Clinical Resource: Antimicrobial stewardship: What it means in tissue viability
Valerie Edwards-Jones
Emeritus Professor of Medical Microbiology, Essential Microbiology Limited
Pam Spruce
TVRE Consulting
Clinical Resource
Antimicrobial stewardship: What it means in tissue viability
Valerie Edwards-Jones
Emeritus Professor of Medical Microbiology, Essential Microbiology Limited
Pam Spruce
TVRE Consulting
INTRODUCTION
This article is based on a Made Easy workshop held at the Wounds UK annual conference in Harrogate, UK, on the 5th of November 2018, sponsored by ActivHeal®, from Advanced Medical Solutions. The aim of the workshop was to look at what is known so far about antibiotic resistance, how the impact of serious infections can be reduced, and to promote the safe and effective use of antimicrobial dressings.
The workshop consisted of presentations by two speakers: Valerie Edwards-Jones (Emeritus Professor of Medical Microbiology, Essential Microbiology Limited), who explained some of the facts around antibiotic resistance and the benefits of antiseptics; and Pam Spruce (TVRE Consulting), who presented some examples of antiseptic dressings, which can be used in wound care. Between them, they have 94 years of experience in microbiology and nursing.
ANTIMICROBIAL RESISTANCE: THE FACTS
When Alexander Fleming received his Nobel Prize in 1945 for discovering penicillin, he said: “There is the danger that the ignorant man may easily under-dose himself and by exposing his microbes to the nonlethal quantities of the drug, make them resistant.” Surveillance around antibiotic resistance over the last 30 years has highlighted an increase, especially in healthcare environments. This is problematic for patients going forward as, if they need treating for an infection or radical surgery, there is a grave possibility that there will not be antibiotics to treat them effectively.
Many bacteria that can cause healthcare-associated infections are becoming multi-drug resistant. Penicillin, widely viewed as the ‘favourite’ antibiotic, was introduced in 1939 but by the mid-1950s there was already some resistance seen. Valerie Edwards-Jones explained a handful of interesting, yet alarming facts around antibiotic resistance, which include the following:
- There have been no new classes of antibiotics for 25 years
- Current antibiotics may become useless within the next two decades
- Things as common as strep throat or a child’s scratched knee could once again kill
- A post-antibiotic era means an end to modern medicine as we know it
THE SCOPE OF THE PROBLEM IN THE UK
According to a study undertaken into antibiotic prescribing by general practitioners, it was shown that 30% of patients received one course of antibiotic treatment per year (Dolk et al, 2018). The median prescribing rate was 626 prescriptions per 1,000 patients. Antibiotic prescribing rates were highest in the elderly, but the study also showed that 46% of prescriptions were linked to respiratory infections, 23% linked to urogenital tract infections, and 16% were for skin and wounds. Interestingly, in almost one-third of all prescriptions, no clinical justification was documented. Antibiotic prescribing rates varied substantially between GP practices, suggesting that there is a potential for antimicrobial stewardship and for prescribing to be reduced in at least some practices (Dolk et al, 2018).
In relation to wound care, a Norwegian study (Gurgen, 2014) reviewed 105 patients referred to a hospital for treatment from primary care. The study found that 75.1% of patients had received antibiotics before referral, 53.3% had systemic antibiotics and 8.6% had local antibiotics. Swabs were taken in 31.4% of patients. Staphylococcus aureus was the most common pathogen (43%) but antibiotics had been administered in 84.5% of these cases and had not solved the problem, with the patient still having to be admitted to hospital. When the hospital’s Infection Control and Tissue Viability Team examined those patients and their records, they would only have prescribed antibiotics in one case (0.9%).
HOW IS IT BEING TACKLED?
Antimicrobial resistance (AMR) is a worldwide problem and not just limited to the UK or the USA. Across Europe and in the developed world, antimicrobial stewardship programmes have been instigated because of the problem. AMR is now being tackled on a global scale, because the impact on the world economy is vast. Part of this growing stewardship involves looking at how antimicrobial drugs can be used more effectively to reduce the rise of resistance, as well as treating individual patients. We now have greater molecular biology technologies (such as PCR and DNA technologies), and computerisation helps us analyse further and predict what might happen.
In the same way that predictive modelling was used to predict how cholera would affect certain countries, the same is being undertaken now for antimicrobial resistance. More money is being invested into boosting new drug developments (£275 million has been released in the UK in the last 2-3 years), and alternatives to antibiotic treatments are being studied: for example phage therapy, which was used in Russia in the 1950s and 1960s. Overall, there is widespread international action spanning drug regulation, and drug use across humans, animals and the environment.
WHAT COULD WE HAVE DONE DIFFERENTLY?
It goes without saying that antibiotics could have been monitored more effectively, but combination therapy could also have been used more widely, as well as cycling antibiotics. For example, penicillin could have been used in serious infections for three days, and another (such as Clarithromycin) could have been used for two days. Dosage is also important, and an alternative option could have been to prescribe higher dosages for shorter time periods, particularly as many people stop taking antibiotics once they start to feel better and do not finish the complete course.
Public Health England is promoting and monitoring judicious use of antimicrobials. There is strong evidence to show that antimicrobial stewardship will reduce inappropriate prescribing, improve patient outcomes, and will be more cost effective. A ‘whole society approach’ is needed with commissioners, providers and patients all to come on board and start working towards the same goals.
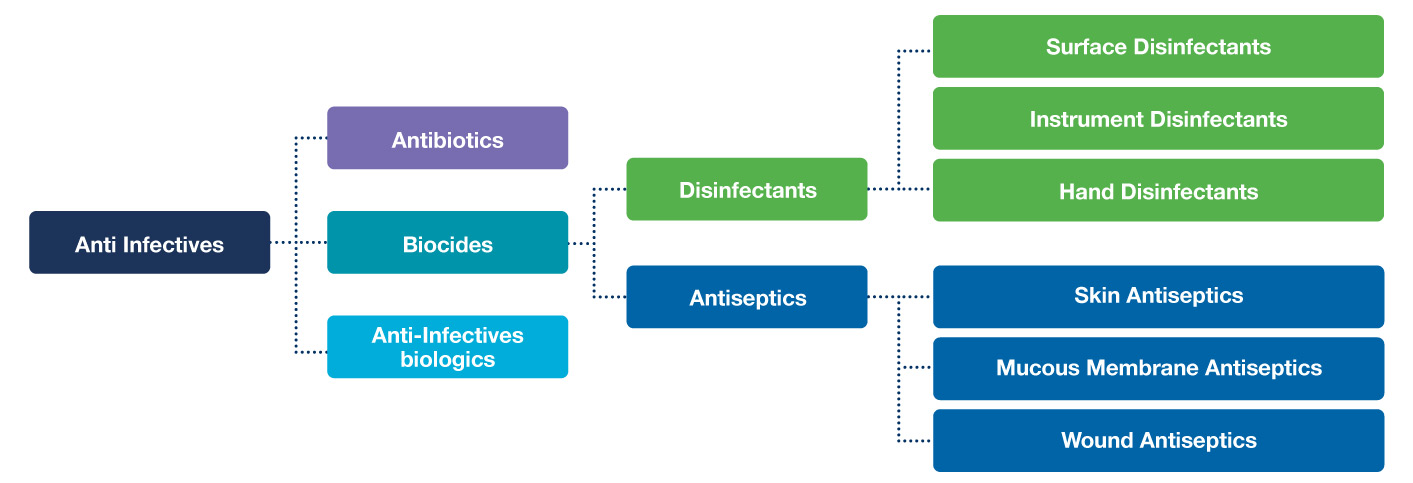
Figure 1. Classification of antimicrobial compounds
ANTIMICROBIAL STEWARDSHIP AND THE USE OF ANTISEPTICS
Antiseptics are being used more and more around the home, in infection control and prevention, and in our everyday lives. We therefore need to consider the following points in relation to antiseptics and wound care:
- Which antiseptics are available for wound care?
- How do we monitor their usage?
- How do we know they work?
- What is their speed of kill?
- Who determines levels applied to wounds?
- What levels should be available in dressings?
- How often should an antiseptic be used and for how long?
- What is the difference between antibiotics and antiseptics? (Roberts et al, 2017)
An antimicrobial is an anti-infective agent – it is something that will have an effect on a microbe. Figure 1 shows the classification of antimicrobial compounds, broken down into antibiotics, anti-infective biologics and biocides. Antibiotics are anti-bacterials and where the most resistance is seen, increasing year on year. Anti-infective biologics use enzymes (e.g. lactoperoxidase) generated in the same way as in a macrophage. Biocides are chemicals that kill micro-organisms by various modes of action. Examples of biocides include surface disinfectants – which cannot be used to treat the human body – but also antiseptics, which are part of the same group but are far less toxic to the human body.
WHAT ANTISEPTICS ARE COMMONLY AVAILABLE FOR WOUND CARE?
WHAT ANTISEPTICS ARE COMMONLY AVAILABLE FOR WOUND CARE?
Common antiseptics available for wound care are silver, polyhexamethylene biguanide (PHMB), honey, iodine and enzymes (Butcher, 2012).
// SILVER
Silver affects DNA, binds to cytoplasmic enzymes and makes them inactive in the bacterial cell, and it also binds to the membrane. There has been some resistance seen, but not in clinical patients.
// PHMB
PHMB is a positively charged molecule that sticks to the negatively charged membrane in the bacterial cell wall until it disrupts the whole cell envelope; as Valerie describes, ‘it punches a hole in it’.
// HONEY
Honey is a good antimicrobial agent because of the high sugar content and it also contains hydrogen peroxide. Manuka honey is particularly effective as it contains terpenoids.
// IODINE
Iodine is an oxidising agent and excellent for skin preparation, although it is often not suitable for chronic wounds where there are high levels of organic matter.
// ENZYMES
Enzymes where interaction of a stabilised enzyme and substrate found in macrophages produces oxygen radicals and lactoperoxidase, which are antimicrobial.
CHALLENGES WHEN USING ANTISEPTIC DRESSINGS
Pam Spruce explained that there are many differences when using antiseptics compared to antibiotics, and whilst considering these differences, it is a good time to reflect on how we worked in the past. It is clear that antibiotics were widely recommended to patients even if they were not needed, and this has contributed to some of the problems today. Supportive therapies were very limited, compression bandaging was not available, and pressure-relieving equipment was scarce. Everything was dependant on antibiotics to kick start the healing process. In the past, a patient may have presented with a non-healing wound and would have been prescribed an antibiotic by the medical practitioner. The instructions for their use would be very prescriptive of how frequently to take the antibiotics and the duration of the course.
While antiseptic dressings are now widely used for suspected and diagnosed infection, their use can be very much what Pam described as a ‘finger in the air’ exercise. Clinicians have a free rein and judgement on which agent to use, how often to change the dressing, and how soon to start and when to stop treatment. Therefore, developing and adhering to best practice is essential.
BEST PRACTICE IN WOUND CARE
Practice has changed rapidly in response to the demand for effective infection prevention and management. The effective use of antiseptic dressings can be complex and needs to be part of a process, which includes a good assessment, biofilm-based wound care, knowledge of the agent being used and how it works, and knowing what interferes with it and how to get the best outcome. Pam’s motto is to ‘keep it simple and make it easy’ when undertaking wound assessment. Using a standardised approach is very important, and she referred to the Ropper Lothian Ladder assessment tool (Ropper, 2018), which has been implemented in Scotland and points clinicians towards looking at the signs and symptoms of clinical infection and using the best interventions to get the best outcomes.
As Figure 2 shows, one of the main aims of wound care is to reduce the biofilm burden, and for effective and speedy debridement to take place. Some of the best ways to debride are sharp debridement, mechanical debridement and larval therapy. This should be followed by vigorous cleansing with an effective cleansing agent. If the cleansing agents are used properly and manufacturers’ instructions followed, they can reduce the biofilm burden. Antiseptic dressings are more effective on a ‘clean’ wound bed. Therefore the wound bed must be prepared properly
It is then important to select the most appropriate dressing, which contains the relevant antiseptic. There are a number of different types of dressings available, which are impregnated with either silver, PHMB, honey or iodine. The type of dressing used should depend on a number of factors such as the wound type, size, location and exudate level. The frequency of dressing change should be dependent on the infection status.
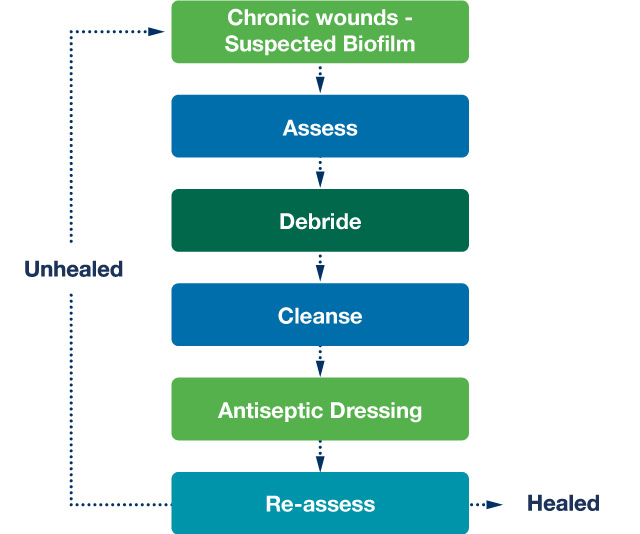
Figure 2. Biofilm-based wound care (Adapted from Bjarnsholt et al, 2016)
PROPERTIES OF A CARRIER DRESSING
The function of a good carrier dressing is to enhance the contact of the antiseptic agent with the wound bed. It is to also maintain the correct moisture level at the wound interface by managing exudate effectively. There are a number of dressings which can effectively carry antiseptics, whilst providing other properties to facilitate wound healing. For example, when an absorbent foam dressing is used, this usually has a breathable outer membrane, which supports fluid handling and prevents maceration. This is usually showerproof for the patient’s personal hygiene and quality of life, and provides a bacterial barrier to the wound. Any carrier dressing should complement other adjunctive therapies – for example, compression bandages – and not inhibit their effectiveness. Three dressings have been developed by ActivHeal® (Advanced Medical Solutions), which incorporate the use of different antiseptics in wound care.
THE TWO DRESSINGS
ACTIVHEAL® AQUAFIBER™ AG
This incorporates silver technology within a fibre dressing and contains 2.3% of ionic silver. It is broad-spectrum, with a sustained silver release and a high wet strength, so it can be used as a cavity dressing and maintains its integrity (data on file).
ACTIVHEAL® PHMB FOAM
This absorbent foam dressing contains PHMB and is in the form of an antimicrobial hydrophilic polyurethane foam pad. It maintains a moist wound environment ideal for wound healing, whilst absorbing excess exudate to prevent peri-wound maceration. It is also broad-spectrum, with sustained antimicrobial release and is effective for up to seven days, allowing the patient to continue everyday activities. It also provides a waterproof bacterial barrier (data on file).
Using the information we have gained from best practice, and what we know about these products and their properties, a framework has been constructed to work out which dressing is most appropriate to use in individual circumstances. Using the algorithm (Figure 3), information from the patient and wound assessment can be used to determine the most appropriate antiseptic agent and dressing for that individual. For example, the age of the patient must be considered, as silver is often discouraged as a first-line antiseptic in children, while PHMB can be used.
Whilst both silver and PHMB can be used for all genders, this may change if a female is pregnant or breast feeding, when silver should be used with caution. In relation to the wound assessment, wound pain is a key factor. Many wounds are painful and the patient may not be able to tolerate a silver dressing, which can cause transient stinging. PHMB might be a better option in these circumstances as it has been shown in a number of clinical studies to reduce wound pain. Location and depth of the wound must also be considered. For example, a flat dressing may not be the best option for a cavity wound, so ActivHeal® AquaFiber™ or Silgen® Ag might be more appropriate as a cavity filler, used with a secondary dressing such as a foam. The duration of use for these dressings is important. A good measurement of effective treatment is the ‘2-week rule’, using 2 weeks as a review period to see whether the wound is progressing. After this period, if progress has not been made, alternatives should be considered.
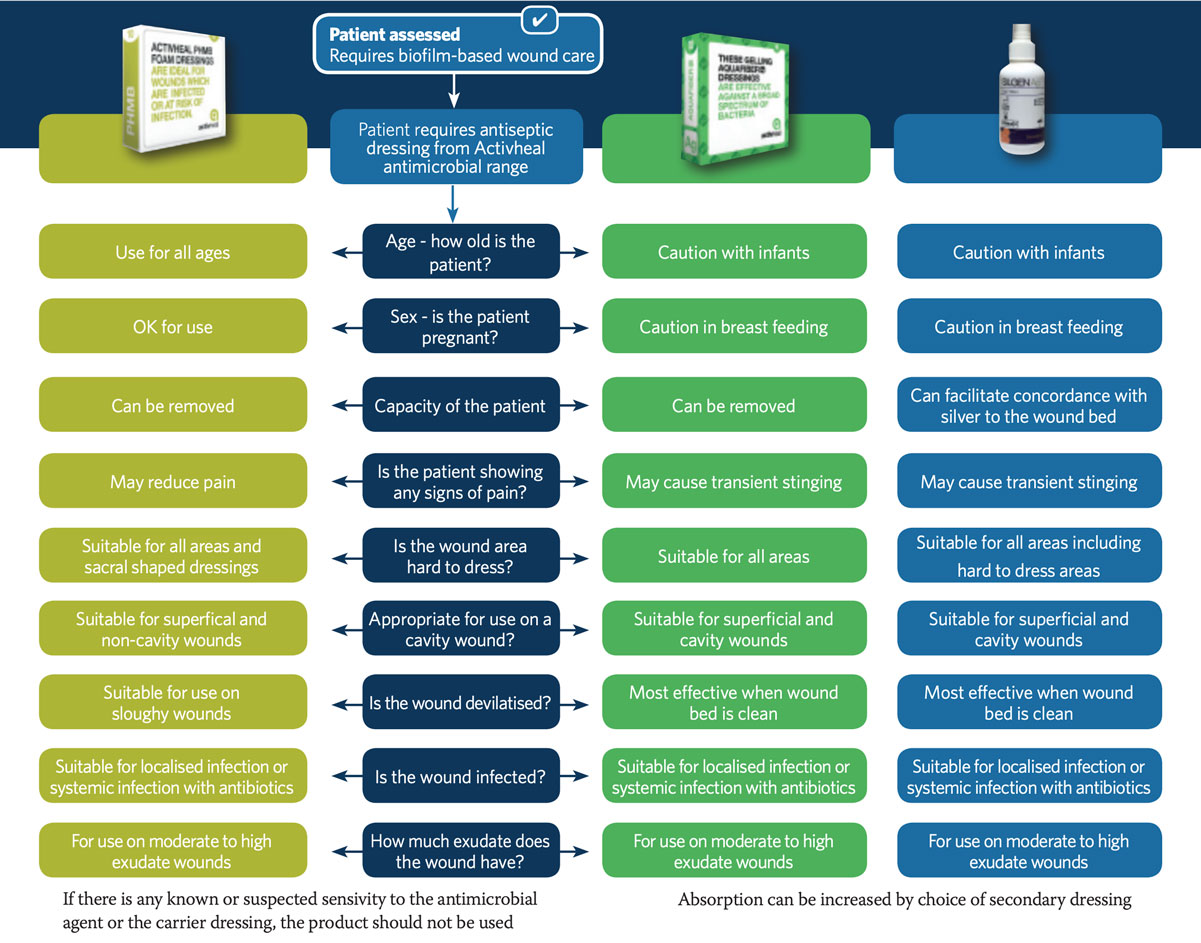
Figure 3. Proposed algorithm for antiseptic dressings
Made Easy Sessions – Valerie Edwards Jones & Pam Spruce from AMS – Branded on Vimeo.
NEXT STEPS
WHAT ANTISEPTICS ARE COMMONLY AVAILABLE FOR WOUND CARE?
- Education will aid effective decision-making
- Knowledge of antiseptics and dressing properties is vital
- Knowledge of when, what and how to apply is key
- Effective monitoring is crucial to guard against future resistance
References
Butcher M (2012) PHMB: an effective antimicrobial in wound bioburden management. British Journal of Nursing 21(12): S16-21
Dolk FCK, Pouwels KB, Smith DRM et al (2018) Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions? Antimicrobial Chemotherapy 73 (suppl 2) s2-10
Bjarnsholt T, Cooper R, Fletcher J et al (2016) World Union of Wound Healing Societies (WUWHS), Florence Congress, Position Document. Management of Biofilm. Wounds International
Gurgen M (2014) Excess use of antibiotics in patients with non-healing ulcers. EWMA Journal 14; 17-22
Roberts CD, Leaper DJ, Assadian O (2017) The role of topical antiseptic agents within antimicrobial stewardship strategies for prevention and treatment of surgical site and chronic open wound infection. Adv Wound Care 6(2): 63-71
Ropper R (2018) The Scottish Ropper Ladder for infected wounds: a 5-year journey from concept to national tool. Wounds UK 14(4): 30-5
CONTACT US FOR MORE INFORMATION
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd




