Clinical Resource: Clinical evaluation of the effect of ActivHeal Aquafiber® Ag dressing
- ACTIVHEAL® AQUAFIBER AG DRESSING
- CLINICAL EVALUATION
- EXUDATE MANAGEMENT
- MOIST WOUND ENVIRONMENT
- HIGH GELLING FIBRE TECHNOLOGY
DONNA WELCH
Advanced Podiatrist, City Healthcare Partnership, Doncaster
LYNNE HEPWORTH
Lead Tissue Viability Nurse, South West Yorkshire Partnership Foundation Trust, Barnsley
SIMON BARRETT
Tissue Viability Lead, Humber NHS Foundation Trust, Hull and the East Riding of Yorkshire
JOANNE OVERFIELD
Tissue Viability Nurse, Humber NHS Foundation Trust, Hull and the East Riding of Yorkshire
REBECCA FORDER
Clinical Research Manager, Advanced Medical Solutions, Winsford
Clinical Resource
Clinical evaluation of the effect of ActivHeal Aquafiber® Ag dressing
- ACTIVHEAL AQUAFIBER AG DRESSING
- CLINICAL EVALUATION
- EXUDATE MANAGEMENT
- MOIST WOUND ENVIRONMENT
- HIGH GELLING FIBRE TECHNOLOGY
DONNA WELCH
Advanced Podiatrist, City Healthcare Partnership, Doncaster
LYNNE HEPWORTH
Lead Tissue Viability Nurse, South West Yorkshire Partnership Foundation Trust, Barnsley
SIMON BARRETT
Tissue Viability Lead, Humber NHS Foundation Trust, Hull and the East Riding of Yorkshire
JOANNE OVERFIELD
Tissue Viability Nurse, Humber NHS Foundation Trust, Hull and the East Riding of Yorkshire
REBECCA FORDER
Clinical Research Manager, Advanced Medical Solutions, Winsford
The management of exudate is an essential requirement in wound care. It is important that an optimal moist environment is created and the surrounding skin protected from the risk of maceration and subsequent risk of infection. Selected antimicrobial dressings for wounds aim to manage exudate levels, maintain a moist wound environment, aid progression and reduce any signs of infection. This article reports on how the ActivHeal® Aquafiber Ag dressing performs on a diverse range of patients with complex infected and non-infected wounds.
HIGH GELLING FIBRE TECHNOLOGY
The term “fibre” dressing is used to describe products manufactured using alginates or carboxymethylcellulose products which are also known as hydrofibers. These dressing products have similar uses in clinical practice, in that they are high gelling fibres used primarily to absorb wound exudate. In a study by Rivas et al (2014) who conducted a trial on 48 patients distributed between a silver hydrofibre and calcium alginate concluded that patients’ outcomes in terms of healing times was not significantly different when they were compared to each other. When both dressings become moistened they retain the exudate forming a gel. As such they are able to assist in the debridement of soft slough. Hydrofibre and alginate dressings are frequently used because they are easy to use and are effective at managing exudate (Griffin, 2014). Alginates have been used within the wound care environment since the early 1940s (Clarke, 2012) and are considered to be versatile dressings, particularly for the management of wounds where there is excess exudate. Alginates like hydrofibre are applied to the wound as a fibre sheer, rope or ribbon, which provides an ideal moist environment required to support wound healing (Clarke, 2012; Hedger, 2014). Both alginates and hydrofibers are chosen for their ability to absorb exudate, creating a gel for ease of removal and ensuring it is undertaken without damaging the wound bed. They can be used on sloughy wounds to aid and facilitate autolytic debridement. They can achieve this by creating a warm, moist environment as they form a gel within the wound bed on contact with wound exudate (Hedger, 2014).
SILVER ANTIMICROBIAL DRESSINGS
Silver is a broad spectrum agent that is effective against a large number of gram positive and gram negative microorganisms, many aerobes and anaerobes and several antibiotic strains such as methicilin resistant Staphylococcus aureus (MRSA) and Vancomycin resistant Enteroccoci (VRE) (Wounds International, 2012). In addition, in vitro silver ions are thought to affect multiple sites within a negatively charged cell component, e.g. the cell wall, DNA and RNA. This disrupts the function of these cell elements and results in cell lysis and interface with electron transport, enzyme function and cell division (Wounds International, 2012). In addition, in vitro studies using experimental biofilm models suggest that silver may kill bacteria within the biofilm matrix and reduce bacterial adhesion, destabilising the matrix (Chaw et al, 2005, Percival et al, 2008). When choosing an appropriate antimicrobial dressing, the priority should be to regain control of the bacterial growth along with reducing the bacterial load. Clinicians should consider how different antimicrobial products support moist wound healing, manage exudate levels and assist in wound bed preparation (Vowden et al, 2011). Although widespread or indiscriminate use of antimicrobial dressings prophylactically is not advocated, there is guidance to support their use in patients with a risk of infection due to comorbidities, or evidence of a history of recurrent infection. Such patients may be at risk of developing cellulitis, or systemic infection which has important clinical, quality of life and cost implications (Wounds UK, 2011). The International Consensus document (Wounds International, 2012) recommends that silver dressings be used initially for a two week ‘challenge period’. At the end of the two week period, the wound, the patient and the management approach should be re-evaluated.
If after two weeks, the wound has:
- Improved, but there are continuing signs of infection; it may be clinically justifiable to continue to use silver dressings with regular reviews.
- Improved and there are no longer signs or symptoms of infection, the silver dressing should be discontinued.
- Not improved, the silver dressing should be discontinued and the patient reviewed and a dressing containing a different antimicrobial agent initiated, with or without systemic antibiotics (Wounds International, 2012).
| Aquafiber Ag | Aquacel ag | |||||
|---|---|---|---|---|---|---|
| Bacteria | 7 days | 14 days | 21 days | 7 days | 14 days | 21 days |
| MRSE | ||||||
| MRSA | ||||||
| VRE | ||||||
| S.pyogenes | ||||||
| S.epidermidis | ||||||
| E.Coli | ||||||
| C.Albicans | ||||||
| P.aeruginosa | ||||||
AQUAFIBER AG
Advanced Medical Solutions has further enhanced their fibre portfolio with the addition of a fibrous antimicrobial dressing ActivHeal Aquafiber Ag. The ActivHeal Aquafiber Ag dressing is a non woven pad composed of calcium alginate, carboxymethylcellulose (CMC) and ionic silver. The ActivHeal Aquafiber Ag releases silver ions in the presence of wound exudate. As the wound exudate is absorbed the dressing forms a gel, which aids autolytic debridement, whilst maintaining a moist environment for optimal wound healing and allows intact removal without damage to healing tissue. The silver ions released in the presence of wound exudate are an effective antimicrobial agent against a broad spectrum of microorganisms frequently associated with bacterial colonisation and infection of wounds, for up to 7 days, based on in-vitro testing (Figure 3) (Advanced Medical Solutions, 2015). The in vitro assessment has shown that the release of silver ions from ActivHeal Aquafiber Ag is sustained for up to seven days (Figure 3), this suggests that the dressing will have antimicrobial efficacy for 7 days (Data on file, Advanced Medical Solutions, 2015).
ActivHeal Aquafiber Ag antimicrobial wound dressing can also help to reduce the odour of infected wounds as a result of its effective antimicrobial properties. The use of topical antiseptic and antimicrobial agents can impact on the bacterial burden and help to reduce the overall bioburden and reduce odour (European Wound Management Association [EWMA], 2006) ActivHeal Aquafiber Ag dressings are indicated for use in the management of moderate to heavily exuding partial to full thickness wounds; post operative wounds, trauma wounds (dermal lesions, trauma injuries or incisions), leg ulcers, pressure ulcers, diabetic ulcers, graft and donor sites, cavity wounds and superficial and partial thickness burns. ActivHeal Aquafiber Ag dressing can be used under medical supervision, in the management of infected wounds or wounds in which there is an increased risk of infection. The ActivHeal Aquafiber Ag dressing has been shown in a number of laboratory studies to have good antimicrobial activity and high absorbent capacity. It has shown to be well tolerated and is suitable for a wide range of wounds offering:
- High absorbency and total fluid handling capacity (Figure 1)
- High wet strength (Figure 2)
- Good antimicrobial activity against many common wound pathogens, including multi resistant organisms (Figure 3)
CLINICAL EVALUATION
A clinical evaluation of the ActivHeal Aquafiber Ag dressing was undertaken at four sites within the community setting. The study was a product evaluation where the use of the dressing was observed within standard practice, and no other changes were made to the wound care pathway. The aim of the study was to assess the overall clinical performance of the ActivHeal Aquafiber Ag dressing when used in the management of acute and chronic wounds and how it contributed to wound progression and managed clinical signs of infection. The patients were not randomised to treatment and no additional interventions were made to standard care. As such, ethical approval was not required, although organisational and patient consent processes were undertaken. Patient confidentiality was also maintained.
As the Aquafiber ActivHeal Ag dressing is used as a primary dressing, the evaluation included the use of the ActivHeal Aquafiber Ag dressing alongside other prescribed dressings used as a secondary dressing. These would be measured in terms of wound outcomes, which included wound size, wound bed status, exudate levels, peri wound skin condition, management of clinical signs and symptoms of infection and wound pain. Dressing performance was evaluated by ease of use, conformability to the wound; patient comfort during removal and wear, clinicians’ satisfaction in regards to ease of application and removal, and its ability to stay in place if the patient showered. The timescale for the evaluation was for a maximum of 10 weeks, although the dressing could be discontinued if the wound healed, was changed to implement a different treatment or if the patient requested the discontinuation. Patients over 18 years of age were included in the evaluation, if they were assessed suitable for a Silver fibre dressing; they were only excluded if they could not give informed consent or had suspected allergies to any of the dressing components. An initial assessment of each patient was undertaken and recorded, during which time the age, sex, wound type, comorbidities, past medical history of the patients were established, including wound type, duration, wound bed status, exudate level; peri wound skin condition, pain, and if the wound showed clinical signs and symptoms of infection (erythema, heat, oedema, abnormal discharge, increased level of exudate, pyrexia, friable tissue, and wound breakdown) as well as previous treatment were also recorded. At each dressing change, the wound assessment was repeated, as well as the reason for the dressing change and performance of the dressing in terms of comfort, ease of application and removal, and additional products used. At the end of the evaluation, the clinicians were asked to rank the overall performance as either very satisfied, satisfied or not satisfied in regards to managing clinical signs and symptoms of infection, managing exudate, maintaining a moist environment, wound progression, ease of use, conformability of the dressing, patient satisfaction and overall assessment of the dressing.
All data was recorded in a standardised form, and a simple analysis was planned where no statistic methods were employed.
RESULTS
WOUND PROGRESSION AND REDUCTION IN SIZE
At the end of the evaluation the Aquafiber Ag dressing was used on all the indicated wound types and 83% of the wounds treated improved (n=31) and showed wound improvement, progression and healing, 3% of the wounds (n=1 healed), and 8% (n=3) showed no wound progression with the wound sizes remaining the same however; the tissue types and wound aetiology showed improvement with a reduction in the percentage of necrotic and sloughy tissue in the wound bed. Of all wounds, 6% (n=2) did not have final measurements, so could not be counted. However, they had shown progression over the course of the evaluation when the measurements had been recorded (Figure 4). No wound sizes increased and there were positive changes and clear wound progression even if it was just removal of devitalised tissue.
At the start of the evaluation 11% (n=4) had necrotic tissue, 5% (n=2) had sloughy tissue, 8% (n=3) had both necrotic and granulating tissue, 68% (n=25) had both sloughy and granulating tissue, and 8% (n=3) had granulating tissue. At the end of the evaluation 0% had necrotic tissue, 5% (n=2) necrotic, sloughy and granulating tissue, 0% had necrotic and granulating tissue, 0% had sloughy tissue, 54% (n=20) had sloughy and granulating tissue, 11% (n=11) had granulating tissue, 3% (n=1) had granulating and epithelising tissue and 11% (n=5) had epithelial tissue (Figure 5). The results show that the dressing met the objective of over 70% of wounds showing improvement in wound bed condition (reduction in unhealthy tissue slough and soft necrosis, increase in granulation and epithelial tissue) with 83% (n=31) of the wounds improving and 3% (n=1) being healed. The results show that the ActivHeal Aquafiber Ag is; enabling wound progression and providing a systematic approach to removing the barriers to healing and enhancing the effects of advanced therapies (Dowsett, 2005).
Based on the data, the mean wound length and width were calculated to be 3.79 cm and 1.73 cm and the depth as 1.1 cm at the start of the evaluation and 2.3 cm and 1.5 cm and the depth 0.98 cm at the end of the evaluation. Measuring a wound at the start of the treatment is seen as best practice to enable accurate assessment of the impact of a clinician’s intervention (Dowsett, 2005). Subsequent measuring can identify whether or not a wound is failing to heal or deteriorating, as the edge of the wound will not epithelise unless the wound bed is well prepared. The data demonstrates a reduction in the mean length and width of the wound at the end of the evaluation, demonstrating wound progression.
Figure 4. Wound progression at the end of the clinical evaluation
Figure 5. Tissue types at the start and end of the clinical evaluation
MANAGING EXUDATE
The exudate levels and peri wound skin condition was also recorded over the duration of the clinical follow up. Exudate levels are hard to quantify and can be subjective depending on the clinical judgement of the clinician assessing the wound (World Union of Wound Healing Societies [WUWHS], 2007). Figure 6 demonstrates that throughout the assessment period there was a trend towards lower levels of exudate, with more patients ending the assessment period either healed or with lower levels of exudate when compared to the start of the evaluation. At the start of the evaluation 30% (n=11) patients had high exudate levels, 57% (n=21) had moderate levels of exudate, and 13% (n=5) had low levels of exudate.
At the end of the evaluation 2% (n=1) had no exudate as the wound had healed, 41% (n=15) had low levels of exudate, 49% (n=18) had moderate levels of exudate and 8% (n=3) had high levels of exudate. There were some patients who still had high levels of exudate at the end of the evaluation as these patients’ wounds had become cavities which can occur when devitalised tissue (necrotic and slough) has been removed. Cavities can generate large amounts of wound exudate therefore the dressings need to be placed into the cavity and contour into the areas of the wound (Timmons et al, 2008) to enable wound healing along with a further absorbent dressing. The results did show in the majority of patients that the ActivHeal Aquafiber Ag dressing successfully and effectively managed exudate, with some wounds having a reduction in the levels of exudate. At the beginning of the evaluation, 16 of the patients on whom the ActivHeal Aquafiber Ag dressing was used as the primary dressing, were observed to have healthy periwound skin. This figure increased to 18 at the end of the study. There was also a reduction in maceration and inflamed skin and some patients were noted to have both symptoms. At the start of the evaluation, 11 patients had macerated periwound skin, reducing to 7 patients at the end of the evaluation (Figure 7). 16 patients had inflamed periwound skin at the start of the evaluation, reducing to 6 patients at the end of the evaluation.
This demonstrates that the ActivHeal Aquafiber Ag dressing is effectively managing wound exudate. Those patients who still had both macerated and inflamed periwound skin were reassessed and moved to a more absorbent secondary dressing along with the ActivHeal Aquafiber Ag dressing. Although gelling fibres can absorb much of the exudate produced by heavily exuding wounds, a more absorbent secondary dressing may be required (Clark, 2012). Once the secondary dressing had been changed there was further improvement to the periwound skin.
Figure 6. Exudate levels at the start and end of the clinical evaluation
Figure 7. Exudate levels at the start and end of the clinical evaluation
CLINICAL SIGNS OF INFECTION
Signs of clinical infection were used to assess the ability of the dressing to control the wound bioburden. The signs and symptoms of clinical infection are defined as erythema, heat, oedema, pain and abnormal discharge, levels of exudate, elevated body temperature, friable tissue, and wound breakdown. At the start of the evaluation 49% (n=18) showed clinical signs of infection and 51% (n=19) showed no signs of clinical infection. At the end of the evaluation 16% (n=6) still showed clinical signs of infection and 84% (n=31) of the wounds showed no signs of clinical infection, there was a 33% reduction in wounds demonstrating clinical signs of infection following the application and use of the ActivHeal Aquafiber Ag dressing on those infected wounds (Figure 8).
None of the wounds showed an increase in the clinical signs of infection, however those wounds that continued to have signs of infection were changed to another type of antimicrobial dressing as good clinical practice dictates, however all wounds within the study were making progress with reduction in devitalised tissue, increase in granulating tissue and the reduction in wound size. The 2-week challenge rule (Wounds UK, 2011) was applied to this study and this determined the duration of use of an antimicrobial dressing. In wounds that are thought to be critically colonised, a topical antimicrobial agent may be considered. The selection of the appropriate wound management products should be done following the assessment of tissue types present, the level of exudate and patient comfort. Failure of the wound to improve after 2 weeks is an indicator to change to a different dressing if signs of infection continue (Wounds UK, 2011).
Figure 8. Exudate levels at the start and the end of the clinical evaluation
PAIN
Pain was measured using a visual analogue scale (VAS) at each treatment visit. Patients were asked to point on the line of the scale to determine what best described the level of pain they had. A score of 0 represented no pain and 10 represented worst pain. At the start of the clinical evaluation 62% (n=23) had pain and 36% (n=16) indicating having no pain. At the end of the clinical evaluation 40% (n=15) still had pain but of less severity and 54% (n=20) had no pain. 6% (n=2) had made no response to this question. The mean pain score for the ActivHeal Aquafiber Ag dressing reduced from 6 at initial assessment to 3 at the end of the clinical evaluation.
PERFORMANCE
At the end of the clinical follow up, the clinicians were asked to rate their overall acceptability of the ActivHeal Aquafiber Ag dressings as being ‘very satisfied’, ‘satisfied’ and ‘not satisfied’. All parameters had levels of satisfaction and met the study objectives and secondary performance requirements of at least 70% of the assessments of each criterion scoring ‘satisfactory’ or ‘better’. (Figure 9). Highlighted performances include:
- Management of exudate 100% (n=37) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Maintaining a moist wound environment 97% (n=36) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Wound progression 97% (n=36) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Reducing signs of infection 94% (n=35) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Ease of use 100% (n=37) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Satisfaction on the conformability of the dressing 100% (n=37) were ‘satisfied’ or ‘very satisfied’.
- Patient satisfaction 95% (n=35) were ‘satisfied’ or ‘very satisfied’.
- Overall assessment of the dressing 100% (n=37) were ‘satisfied’ or ‘very satisfied’.
In respect to exudate management, maintaining a moist wound environment, reducing the signs of infection and wound progression, the above evidence substantiates the safety and performance of the dressing whilst meeting its intended performance of treating patients with infected wounds or those at high risk of infection and translates into positive outcomes for the patient and clinician. The results suggest that the ActivHeal Aquafiber Ag dressing effectively managed exudate levels, maintained a moist wound environment and reduced the clinical signs and symptoms of infection. The ActivHeal Aquafiber Ag dressing also addresses patient’s needs in terms of ease of use, conformability and patient satisfaction and comfort, which again translates into positive outcomes for the patient. The results of the clinical follow up meets the primary and secondary objectives, providing evidence that the ActivHeal Aquafiber Ag dressing had positive healing outcomes and performance for patients with hard-to-heal wounds.
Figure 9. Percentage of clinician and patient satisfaction levels of dressing characteristics
CONCLUSION
Antimicrobial dressings are popular and used frequently in the management of infected exuding wounds, or at risk of infection. The clinical evaluation was limited in that it had uncontrolled patient numbers, however, the results of the clinical evaluation do demonstrate positive endpoints for both the primary and secondary objectives in the cohort of 37 patients. It can be concluded that the ActivHeal Aquafiber Ag dressing is safe, performs well and is efficacious in promoting healing through the management of wound exudate, maintenance of a moist wound environment as well as controlling the wound bioburden in the management of both acute and chronic wounds. The clinical evaluation provides good preliminary evidence of the clinical benefits of the ActivHeal Aquafiber Ag dressing.
CASE STUDY
INTRODUCTION
A 86-year-old female was admitted to a residential care home, following a total hip replacement after falling and fracturing her hip and the development of a category 3 pressure ulcer. She had a previous medical history of a Myocardial Infarction, Transient Ischaemic attacks, Coronary artery bypass graft, Atrial fibrillation and Osteoarthritis. The patient had previously been cared for at home, however she had become less mobile and incontinent of urine and a category 3 pressure ulcer (European Pressure Ulcer Advisory Panel (EPUAP), 2014) on the sacrum developed. Once in the care home the patient was nursed on an alternating mattress and received 2 hourly repositioning. A referral was made to tissue viability as the pressure ulcer had deteriorated to a category 4 to the sacrum, exacerbated by the patient’s incontinence and probable contamination of the wound with urine. A full assessment was undertaken along with a wound swab.
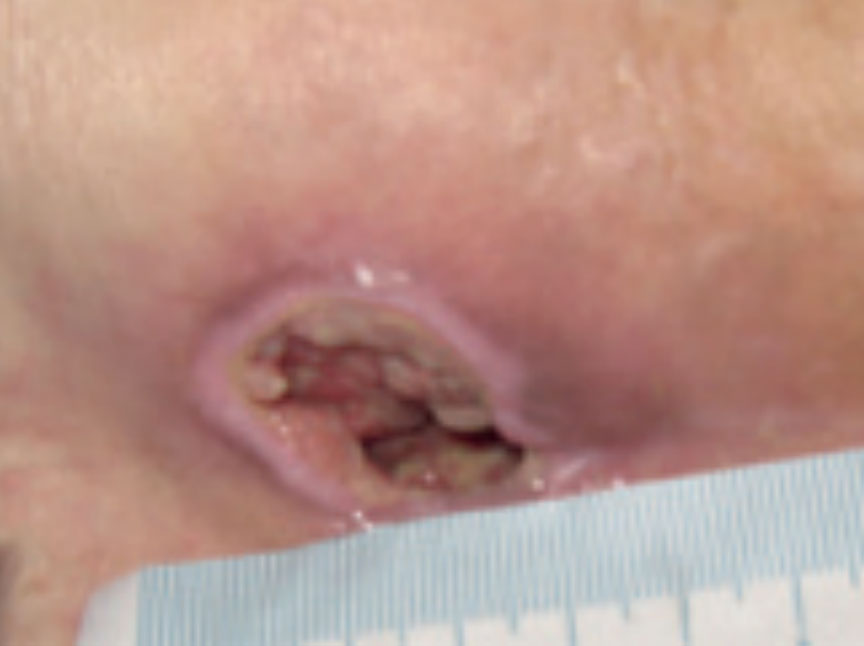
Figure 10. Wound at initial assessment
At initial assessment, the ulcer measured 3.7 cm x 3.2 cm with a depth of 2 cm and the wound undermined downwards towards the rectum by 3.5 cm. The pressure ulcer had 50% slough and 50% granulation tissue and high exudate levels. The peri wound appeared macerated. The pressure ulcer was showing clinical signs of infection of erythema, abnormal discharge and increased levels of exudate along with a VAS score for pain at 10 (10=worst pain imaginable). The wound was showing clinical signs of infection of erythema, heat, pain, high levels of exudate and abnormal odour (Figure 10). A wound swab was taken and sent for culture and sensitivity. The ActivHeal Aquafiber Ag dressing was selected to assist in reducing wound bioburden, absorb levels of exudate, maintain a moist wound environment, facilitate autolytic debridement and promote healing. The ActivHeal Aquafiber Ag was secured using ActivHeal Foam Contact dressing. The patient was also to be nursed on an alternating pressure relieving mattress and being repositioned every 2 hours.
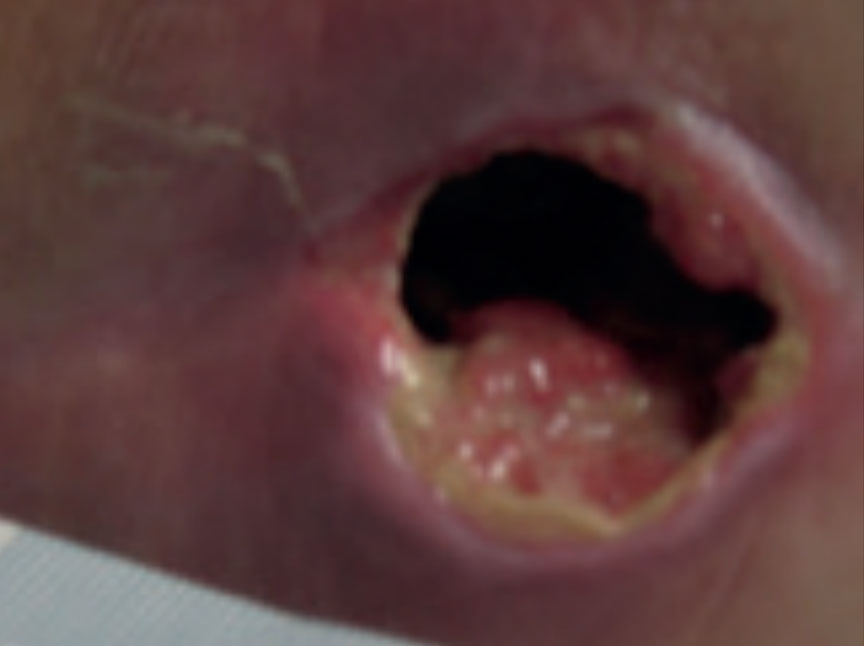
Figure 11. Wound at week 1
Significant progress was noted in the wound, with the wound reducing in size and showing wound progression. The wound measured 2.5 cm x 1.8 cm with a depth of 2 cm. The undermined areas had reduced to 2.5 cm. There was new granulation tissue visible and epithelialisation was evident at the wound margin and a reduction in the amount of slough. Exudate levels remained high however the peri wound was healthy and no longer macerated (Figure 11). The patient was expressing high levels of pain, a VAS score of 10. The wound swab results showed heavy growth of mixed anaerobes and proteus species sensitive to Metronidazole, therefore the patient was commenced on a 7 day course of antibiotics. The same dressing regime was continued as the wound was progressing and reducing in size along with the facilitation of debridement of devitalised tissue despite the high bacterial burden within the wound.
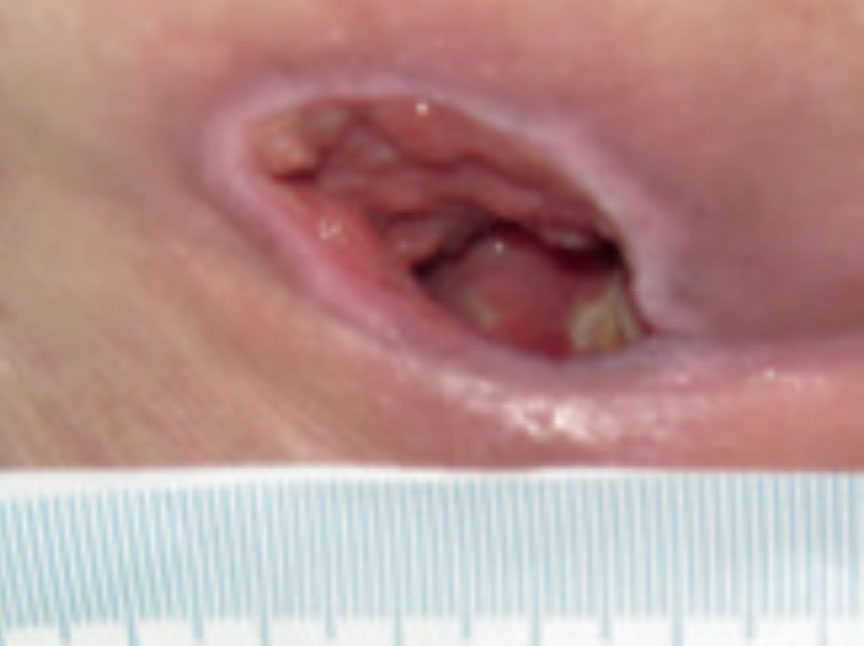
Figure 12. Wound at week 2
Further progress was noted in the wound with a continued reduction in slough, promotion of granulating tissue and epithelial tissue apparent at the wound edges. The wound had 5% sloughy tissue, 90% granulation tissue and 5% epithelial tissue. The wound continued to have high levels of exudate however, the peri wound skin was normal and showed no signs of maceration. The wounds measured 2.3 cm x 2 cm with a depth of 2 cm (Figure 12). The clinical signs of infection were reducing, with only abnormal odour apparent, and the patients pain level had reduced to 6 using a visual analogue scale. The same dressing regime continued.
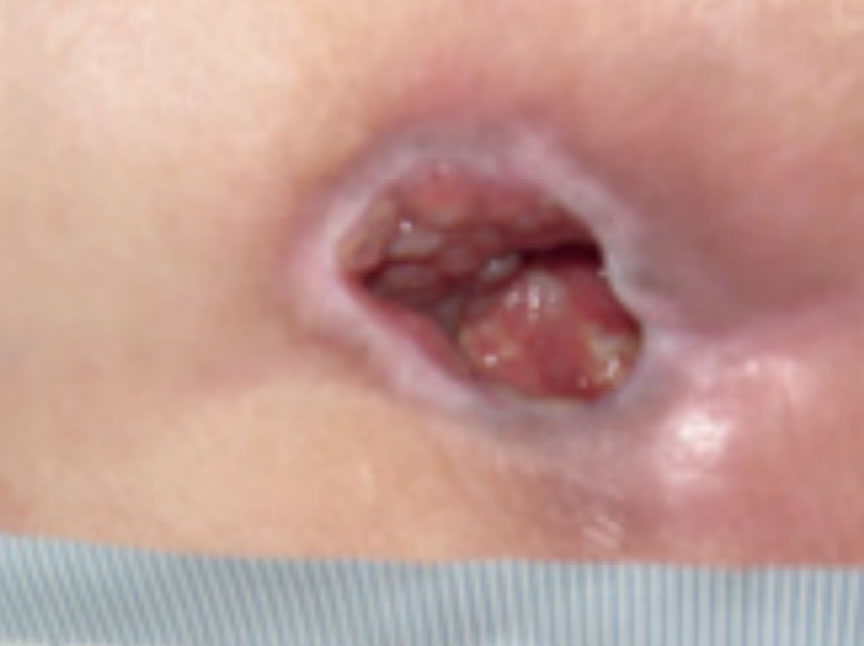
Figure 13. Wound at week 3
At week 3 the wound was reviewed and had continued to improve. The wound measured 2 cm x 2 cm with a depth of 2 cm. The wound no longer contained sloughy tissue but had 95% granulation tissue and 5% epithelial tissue. Exudate levels remained high but all the clinical signs of infection were no longer apparent. The patient’s pain level had also reduced to 0 using a visual analogue scale. Following a full reassessment by the tissue viability nurse, the ActivHeal Aquafiber Ag was discontinued and moved to the use of Negative Pressure Wound Therapy (Figure 13).
CONCLUSION
The ActivHeal Aquafiber Ag dressing was found to be an appropriate dressing in the management of the infected category 4 pressure ulcer with high exudate levels and bacterial bioburden within the wound. Despite this, the wound improved over the 3 weeks of treatment. Granulation tissue was evident in just a few days. Sloughy tissue was also debrided very quickly. The dressing produced very positive patient outcomes. The correct dressing choice in this case enabled the patient to be managed quickly and effectively without an overly long treatment time and assisted in the management of clinical indications of exudate management, reducing wound bioburden along with being safe and acceptable to the patient. The patients pain score reduced throughout the use of the dressing. The case study illustrates the importance of a holistic approach when caring for a patient with a challenging wound and ensuring that the correct diagnosis is made based upon a thorough assessment ensuring good clinical outcomes for the patient.
References
Advanced Medical Solutions (2015) Data on File. LD89-17
Clarke M (2012) Rediscovering alginate dressings. Wounds International 3(2): 24–8
Chaw K, Manimaran M, Tay F (2005) Role of silver ions in destabilisation of intermolecular adhesion forces measured by atomic force microscopy in Staphylococcus epidermis biofilms. Antimicrob Agents Chemother 49(12): 48–59
Dowsett C, Newton H (2005) Wound Bed Preparation: TIME in Practice. Available at: www.wounds-uk.com/pdf/content_9029.pdf (accessed on 18.10.2017)
European Pressure Ulcer Advisory Panel (2014) Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline. Haesler E (ed)
Cambridge Media, Perth, Australia
European Wound Management Association (2006) Association Position Document: Identifying Criteria for Wound Infection. Available at: http://ewma.org/fileadmin/user_upload/EWMA.org/Position_ documents_2002-2008/English_pos_doc_final.pdf (accessed on 18.10.2017)
Griffin J (2014) Effective exudate management improves patient well being. Journal of Community Nursing 28 (5 Suppl): 16–6
Hedger C (2014) Choosing the appropriate dressing: Alginate and Hydrofiber. Wounds Essentials 9( 1): 29–33
Percival S, Bowler P, Woods E (2008) Assessing the effect of an antimicrobial wound dressings on biofilms. Wound Repair Regen. 16 (1): 52–7
Rivas C, Rivas Y, Valero K (2014) Effectiveness of the Dressing with Silver Hydrofiber vs Calci Alginate in Ulcer in Healing in Diabetic Foot. Published Thesis
Timmons J, Cooper P (2008) How to systematically assess a patient with a cavity wound. Wounds UK Vol 4(2): 4–10
Vowden P, Vowden K, Carville K (2011) Antimicrobial Dressings Made Easy. Available at: www.woundsinternational.com/made-easys/view/ antimicrobial-dressings-made-easy-1 (accessed 18.10.2017)
Wounds International (2012) International Consensus Appropriate Use of Silver Dressings in Wounds. An Expert Working Group Review. Available at: www.woundsinternational.com/media/issues/567/files/ content_10381.pdf (accessed on 18.10.2017)
World Union of Wound Healing Societies (2007) Principles of Best Practice: Wound Exudate and the Role of Wound Dressings. A Consensus Document. Available at: www.woundsinternational.com/media/ issues/82/files/content_42.pdf (accessed on 18.10.2017)
Wounds UK (2011) Best Practice Statement: The Use of Topical Antiseptics/ Antimicrobials in Wound Management. Available at: http://bit. ly/2zceoi1 (accessed 18.10.2017)
CONTACT US FOR MORE INFORMATION
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd


