THIS FAST GELLING ALGINATE LIKES WET WOUNDS.
ACTIVHEAL® ALGINATE
THIS FAST GELLING ALGINATE LIKES WET WOUNDS.

ACTIVHEAL® ALGINATE
ACTIVHEAL® ALGINATE IS A PRIMARY SODIUM CALCIUM DRESSING THAT IS USED ON MODERATE TO HEAVILY EXUDING WOUNDS.
FEATURES EXCELLENT ABSORPTION OF EXUDATE // VERSATILE // PROMOTES HEALING THROUGH A MOIST WOUND ENVIRONMENT // HAEMOSTAT // AIDS AUTOLYTIC DEBRIDEMENT // ENCOURAGES GRANULATION WITHIN THE WOUND //
ActivHeal® Alginate is manufactured by processing natural elements found in seaweed to produce felt and rope dressings.
The absorbent alginate fibres in the dressing, gel on contact with the wound fluid and gently conform to the wound surface. Alginates absorb exudate away from the wound whilst maintaining an ideal moist wound environment. The versatility of ActivHeal® Alginate dressing means that it conforms well to different shaped wounds and is ideal for difficult to dress areas such as cavity, to promote healing from within.
performance
The key performance attributes of ActivHeal® Alginate dressings are it’s high absorbency and high wet strength. The charts below demonstrates it’s performance. When ActivHeal® Alginate dressings are applied to an exuding wound the sodium salts present in exudate exchange with calcium in the alginate to form a hydrophilic gel. The high wet strength of ActivHeal® Alginate ensures the dressing remains integral on removal. Alginate fibres are biodegradable1 therefore any residual fibres that remain after a dressing change pose no risk to patient safety. ActivHeal® Alginate fibres are naturally haemostatic. Following absorption of wound exudate the alginate dressing releases calcium ions into the wound which can activate platelets to control minor bleeding2.
The charts below demonstrate its performance against other market leading brands
Absorbency3 (g/100cm2)
Wet strength3 (N/cm)
product range
- EVERYDAY: Alginate Sheet & Alginate Ribbon

indications
ActivHeal® Alginate is indicated for moderately to heavily exuding wounds that are granulating or with areas of slough4:
- Pressure ulcers
- Venous leg ulcers and arterial ulcers
- Diabetic ulcers
- Cavity wounds
- Graft wounds and Donor sites
- Post operative surgical wounds
- Superficial and partial thickness burns
- To control minor bleeding
wound types

Moderate > Heavy Exudate
case study
Mr B is a 55 year old male with type 2 diabetes and peripheral neuropathy. Mr B whilst on holiday had an injury to his foot and didn’t receive appropriate treatment. The patient presented to hospital 20 days after the initial injury. He had been treated with low dose oral antibiotics and non-adherent dressings. He presented with a gangrenous toe with associated osteomylitis. Subsequently the patient underwent surgical amputation of his second and third toes from his left foot. ActivHeal® Alginate dressing was selected and applied as the wound was exudating at a high level.
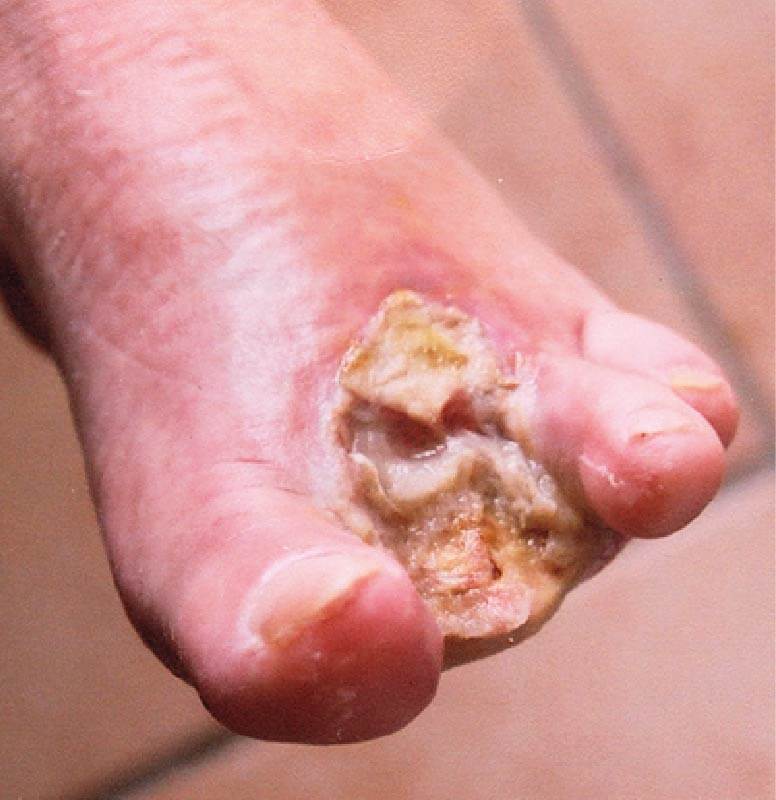
WEEK 1. Following amputation the wound was highly exudating and comprised of 90% slough.
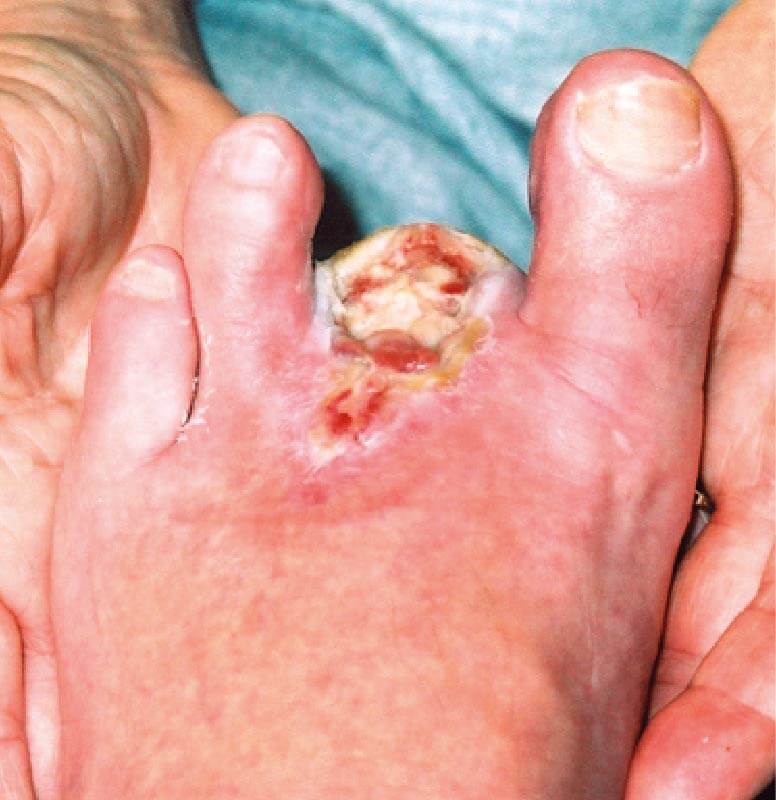
WEEK 4. The wound showed signs of progression with a significant reduction in size and newly formed epithelial tissue was present.
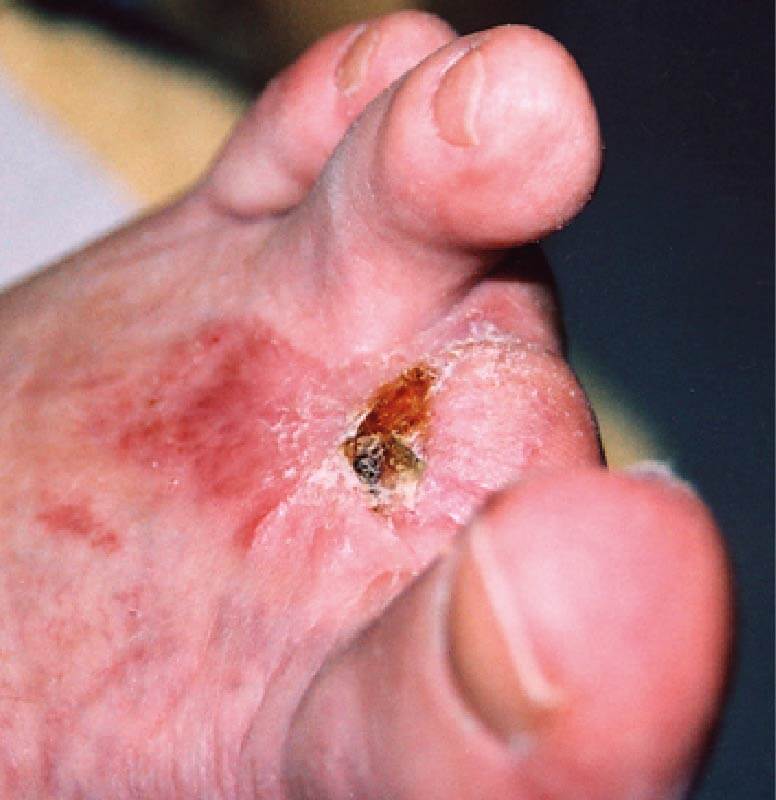
WEEK 9. The wound showed a significant sign of improvement with granulation tissue being observed.
FREE TWO WEEK EVALUATION
ACTIVELY TRY AQUAFIBER ALGINATE FOR TWO WEEKS TO SEE HOW IT DELIVERS A HIGH PERFORMANCE WOUND CARE SOLUTION.
*Health care professionals only
* Required Field

SIZES & CODES
ActivHeal® Alginate is available through a variety of channels.
| ACTIVHEAL® ALGINATE | ||||
|---|---|---|---|---|
| SIZE (CM) | QTY | PRODUCT CODE | NHS SUPPLY CHAIN | DT PIP CODE |
| 5x5 | 10 | 10007432 | ELS139 | 301-6722 |
| 10x10 | 10 | 10007431 | ELS140 | 301-6771 |
| 10x20 | 5 | 10007430 | ELS141 | 301-6789 |
| 2x30 Rope | 5 | 10007428 | ELS142 | 301-6797 |
References
1. Lansdown AB, Payne MJ. (1994) An evaluation of the local reaction and biodegradation of calcium sodium alginate (Kaltostat) following subcutaneous implantation in the rat. J R Coll Surg Edinb. Oct: 39 (5) : 284-8
2. Thomas, S (2000) Alginate dressings in surgery and wound management: Part 3
3. Data on file
4. Morris C (2006) Wound management and dressing selection.
Wound Essentials. Volume 1 page 178-183. Kaltostat is a registered trademark of Convatec. Sorbsan is a registered trademark of Aspen.
1. Lansdown AB, Payne MJ. (1994) An evaluation of the local reaction and biodegradation of calcium sodium alginate (Kaltostat) following subcutaneous implantation in the rat. J R Coll Surg Edinb. Oct: 39 (5) : 284-8
2. Thomas, S (2000) Alginate dressings in surgery and wound management: Part 3
3. Data on file
4. Morris C (2006) Wound management and dressing selection.
Wound Essentials. Volume 1 page 178-183. Kaltostat is a registered trademark of Convatec. Sorbsan is a registered trademark of Aspen.
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd