FLEXIBLE AND BREATHABLE SILICONE FOAM LITE. THIN AND DISCREET DRESSING FOR NON TO LOW EXUDING WOUNDS.
ACTIVHEAL® SILICONE FOAM LITE
FREE TWO WEEK EVALUATION
FLEXIBLE AND BREATHABLE SILICONE FOAM LITE. THIN AND DISCREET DRESSING FOR NON TO LOW EXUDING WOUNDS.
ACTIVHEAL® SILICONE FOAM LITE
FREE TWO WEEK EVALUATION
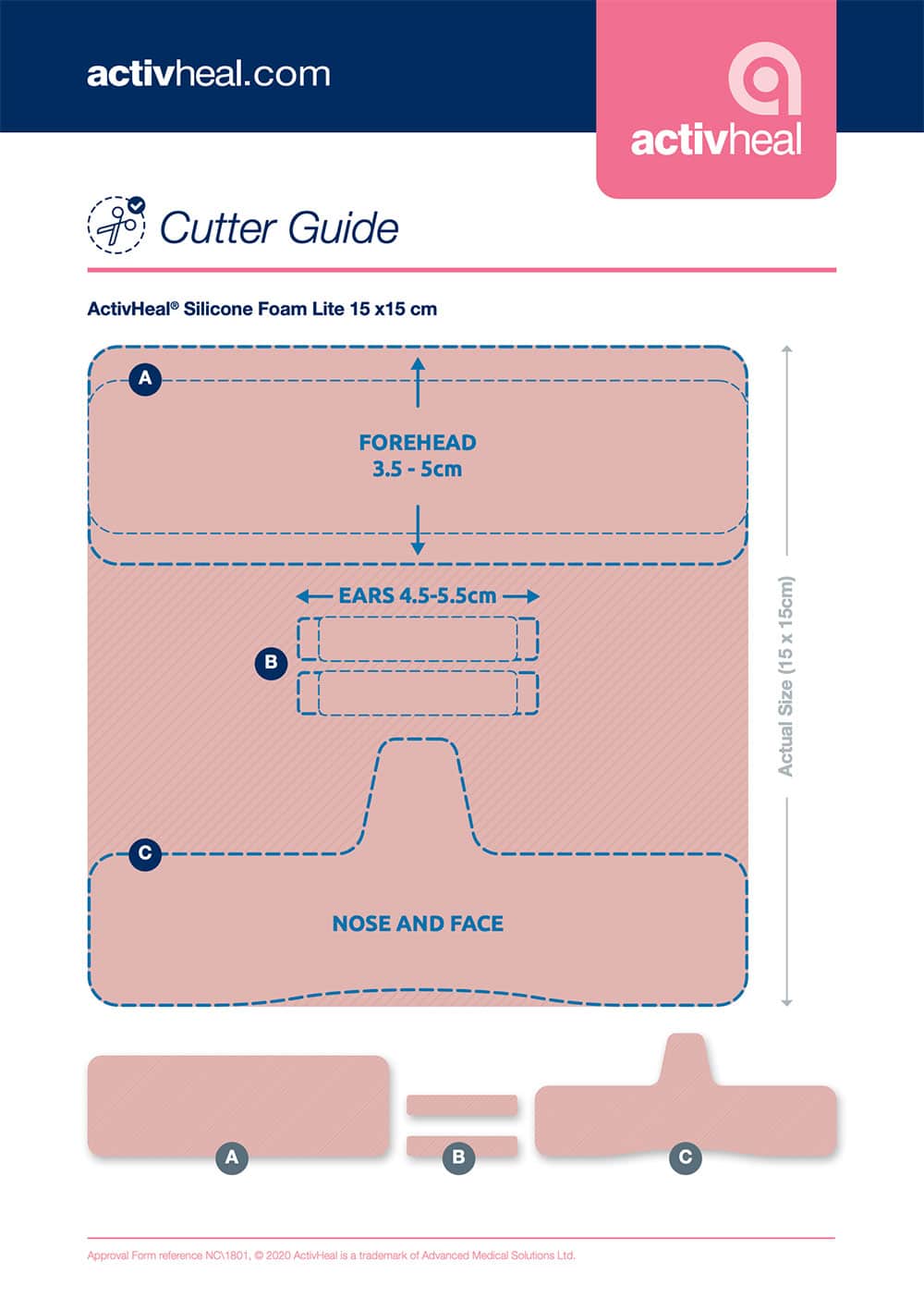
HEALTHCARE WORKERS ARE EXPERIENCING FACIAL SKIN DAMAGE FROM THE CONTINUOUS USE OF PERSONAL PROTECTIVE EQUIPMENT (PPE) SUCH AS GOGGLES AND FACE MASKS. ADVANCED MEDICAL SOLUTIONS ARE HERE TO HELP.
With the use of a silicone foam dressing and our downloadable cutter guide and instructions, the dressing can be adapted to act as an interface between the skin and PPE, minimising pain and trauma.

ACTIVHEAL® SILICONE FOAM LITE THIN, SOFT AND DISCREET HYDROPHILIC FOAM PAD FOR NON AND LOW EXUDING WOUNDS.
FEATURES
- SOFT SILICONE ATRAUMATIC ADHESIVE
- THIN, SOFT AND DISCREET
- WATERPROOF AND BACTERIAL BARRIER
- BREATHABLE BACKING FILM
- SOFT AND COMFORTABLE FOAM PAD
- MINIMAL EPIDERMAL STRIPPING OR PAIN ON REMOVAL
- DRESSING MAY REMAIN IN SITU FOR UP TO 7 DAYS
- CAN BE REPOSITIONED DURING APPLICATION
- CAN BE LIFTED DURING WEAR TIME TO ALLOW OBSERVATION
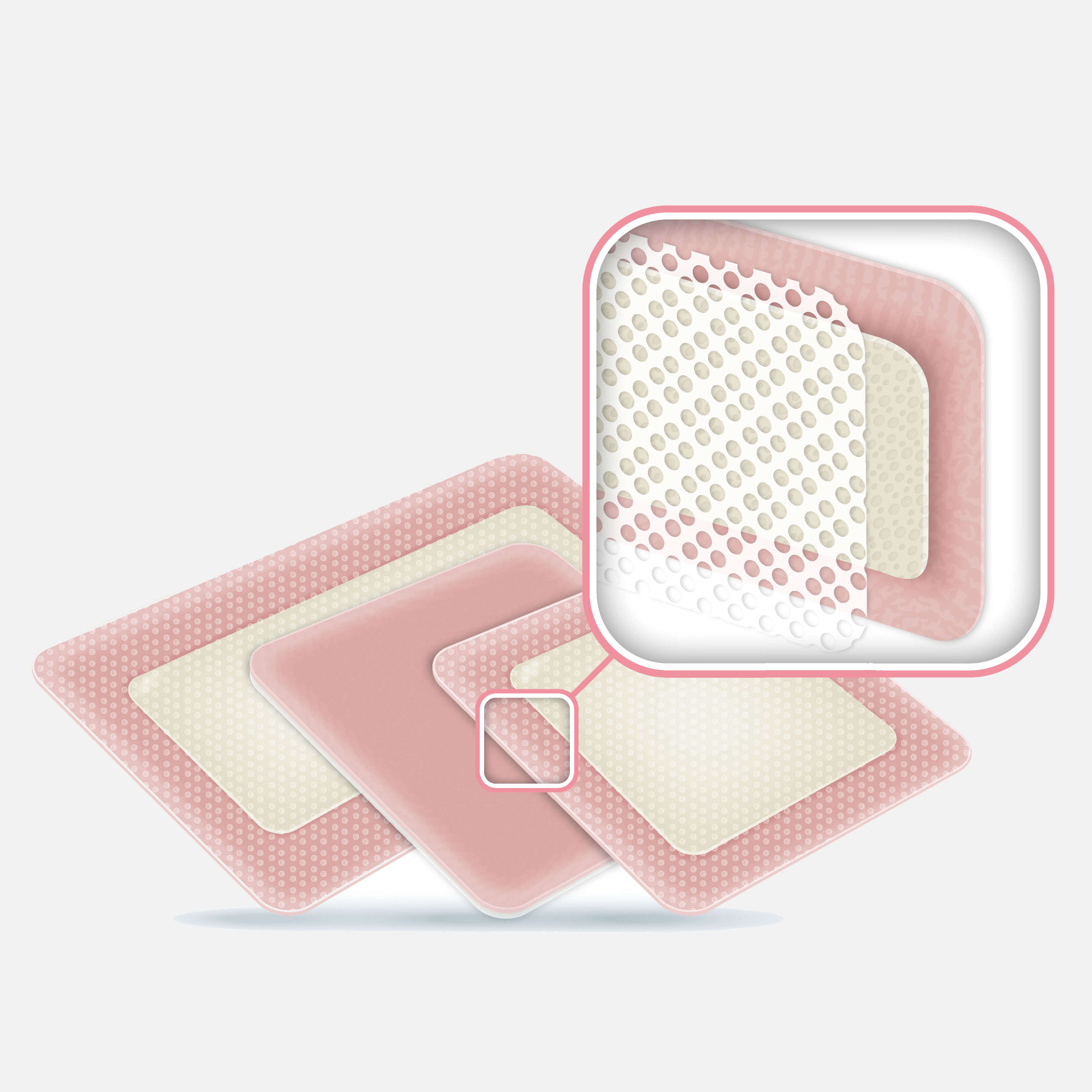
ActivHeal® Silicone Foam Lite dressing is a sterile wound dressing that is soft, thin and discreet and comfortable to anatomical contours. The dressing is breathable and manages non to low exuding wounds. The dressing is easy to remove and provides a gentle but secure adhesion to skin with minimal epidermal stripping and pain during removal.
ActivHeal® Silicone Foam Lite dressing has a trilaminate structure consisting of a hydrophilic, absorbent, polyurethane central foam layer, and a breathable and low friction, polyurethane film outer layer, which provides a waterproof and bacterial barrier. The wound contact layer is a silicone adhesive coated perforated film.
ActivHeal® Silicone Foam Lite dressing manages exudate in non to low exuding wounds and provides a moist wound environment which aids the wound healing process. The dressing helps to minimise maceration and damage to the surrounding skin. The perforated silicone wound contact layer aids atraumatic removal, enables exudate uptake, and prevents ingress of granulation tissue into the dressing.
ActivHeal® Silicone Foam Lite dressing can be repositioned during application or lifted during wear time for observation.
Not made with natural rubber latex.
PERFORMANCE
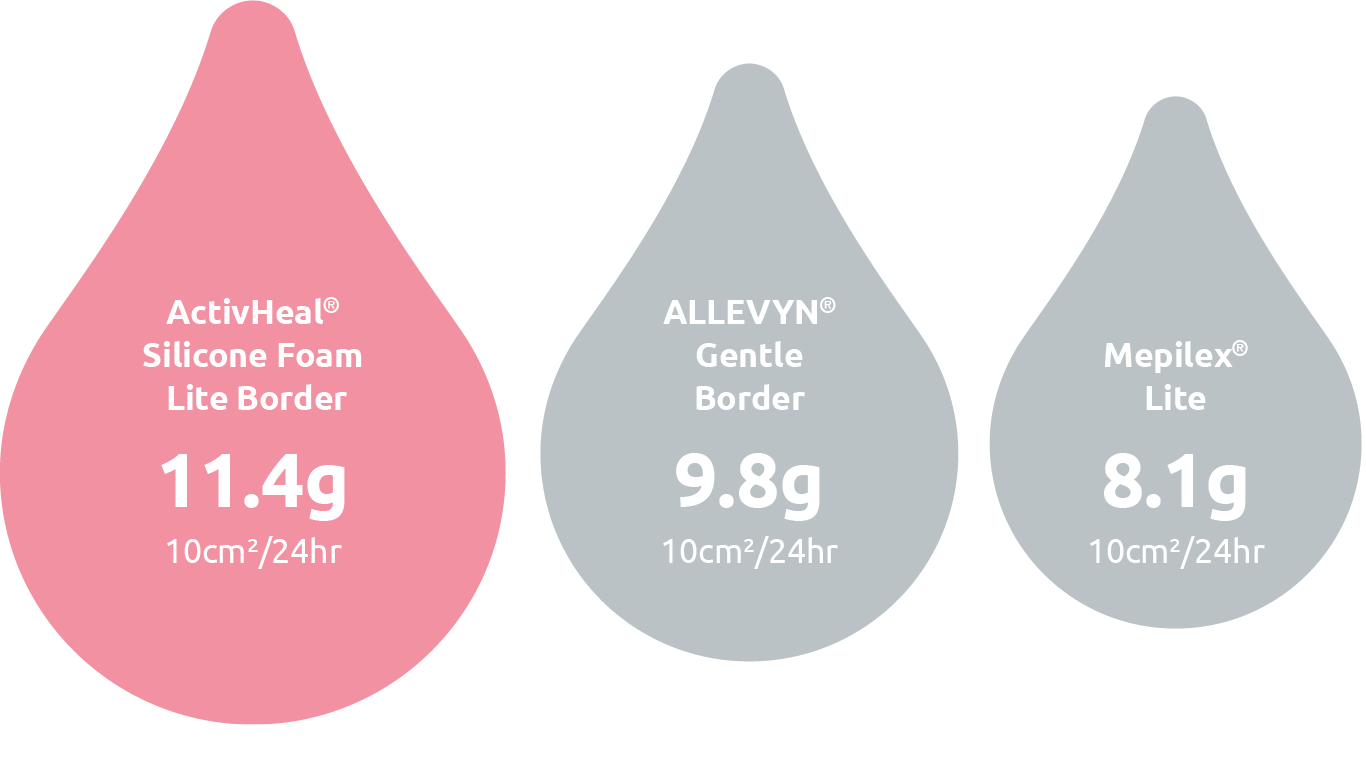
Total Fluid Handling Performance1
Be confident this foam can handle patient exudate.
INDICATIONS
ActivHeal® Silicone Foam Lite dressing is indicated for use in the management of non to low exuding, chronic and acute wounds throughout the healing process. The dressing is indicated for use on the following wounds:
- Pressure ulcers
- Diabetic ulcers
- Leg & foot ulcers
- Post-operative surgical wounds
- Trauma wounds (including lacerations, abrasions, skin tears and blisters)
- Superficial and partial thickness burns
- Radiation damaged skin
The dressing can be used on fragile skin and is suitable for use under compression bandaging.
WOUND TYPES
Non > Low
WOUND TYPES
ActivHeal® Silicone Foam Lite dressings are not indicated for use on the following:
- Do not use on individuals with a known sensitivity to polyurethane films, foams or silicone
- Do not use with oxidising solutions such as hypochlorite or hydrogen peroxide, as these can break down the absorbent polyurethane component of the dressing
- Not for surgical implantation
- Do not re-use in whole or in part, as it may compromise sterility and/or the performance of the dressing
- ActivHeal® Silicone Foam Lite dressing must not be ingested and must be kept away from children and animals
- For use by or under the guidance of a healthcare professional
Post-market clinical evaluation of the safety and performance of ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite dressings
This post-market evaluation assessed the performance of the ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite dressings and gathered feedback from clinicians using both dressings to treat a variety of wounds. The primary outcomes were progression to healing through management of exudate and maintenance of a moist wound healing environment. The evaluation took place at 1 site within Poland, where 53 patients were treated according to ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite dressings’ instructions for use, and standard local practice. Data was collected at every dressing change. The results showed that both ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite Dressings had positive effects on exudate levels, periwound condition and tissue type(s) within the wound bed. The majority of wounds reduced in size during the evaluation period and twelve completely healed. Clinical and patient satisfaction was high. No adverse events were reported. The evaluation shows ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite Dressings used in clinical practice are safe, effective and acceptable for use to both practitioners and patients
FREE TWO WEEK EVALUATION
ACTIVELY TRY SILICONE FOAM LITE FOR TWO WEEKS TO DISCOVER HOW THE FLEXIBLE AND BREATHABLE DRESSING ABSORBS EXUDATE AND KEEPS BACTERIA AWAY FROM WOUNDS.
*Health care professionals only
SIZES & CODES
ActivHeal® Silicone Foam Lite is available through a variety of channels.
| ActivHeal® | SILICONE FOAM LITE BORDER | SILICONE FOAM LITE NON-BORDER | |||||
|---|---|---|---|---|---|---|---|
| SIZE (CM) | QTY | PRODUCT CODE | NHS SUPPLY CHAIN | DT PIP CODE | PRODUCT CODE | NHS SUPPLY CHAIN | DT PIP CODE |
| 5x5 | 10 | 9023687 | ELA1135 | 412-9938 | |||
| 5.5x12 | 10 | 9023694 | ELA1136 | 413-0340 | |||
| 7.5x7.5 | 10 | 9023700 | ELA1137 | 413-0357 | 9023755 | ELA1142 | 413-0423 |
| 10x10 | 10 | 9023717 | ELA1138 | 413-0365 | 9023762 | ELA1143 | 413-0431 |
| 15x15 | 10 | 9023724 | ELA1139 | 413-0373 | 9023779 | ELA1144 | 413-0415 |
| 10x20 | 10 | 9023731 | ELA1140 | 413-0381 | 9023786 | ELA1145 | 413-0407 |
| 10x30 | 10 | 9023748 | ELA1141 | 413-0399 | |||
References
1. P3103R AMS Data on File
DOWNLOADS
CONTACT US FOR MORE INFORMATION
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd