Wound-bed preparation:
A vital step in the healing process
Simon Barrett
Tissue Viability Nurse Specialist, Humber NHS Foundation Trust
Wound-bed preparation:
A vital step in the healing process
Simon Barrett
Tissue Viability Nurse Specialist, Humber NHS Foundation Trust

ABSTRACT
It can impact on the patient by delaying wound healing, increasing the risk of amputation and life-threatening conditions, and reducing quality of life. The use of antibiotics to treat wound infections has decreased due to the increased risk of antibiotic resistance (Swanson et al, 2014). As a result alternative strategies for preventing and managing wound infection have been adopted, including wound debridement and the use of topical antiseptic/antimicrobial agents.
KEY WORDS
- Chronic wounds
- PHMB
- Wound bed preparation
- Infection
WOUND INFECTION
There are classic signs and symptoms that are easily identifiable as wound infection, but not all wounds will exhibit all these signs at any one time. Localised infection is often characterised by the classic signs and symptoms of inflammation, pain, heat, swelling, redness and loss of function (WUWHS, 2008). Additional criteria have been suggested for identifying wound infection, which include:
- Abscess formation
- Cellulitis
- Discharge
- Delayed healing
- Discolouration
- Friable granulation tissue that bleeds easily
- Unexpected pain
- Pain that has changed in nature
- Tenderness, pocketing at the base of the wound
- Bridging of epithelium or soft tissue
- Abnormal smell, and wound breakdown (Cutting et al, 2005)
For healing to proceed it is important to prevent the establishment of localised infection and spreading infection. This may involve the prevention of progression to colonisation or the management of an established localised infection (WUWHS, 2016).
The indiscriminate use of antibiotics has made resistant organisms more prevalent. The use of antibiotics is not recommended because of the development of bacterial resistance, allergic and sensitivity reactions (Sussman et al, 2014).
Wound swabbing is still recommended if there is suspicion of wound infection and is used to determine the most appropriate systemic antibiotic to be prescribed (Sussman et al, 2014). Topical antiseptic/antimicrobial agents such as honey, silver, polyhexamethylene biguanide (PHMB) and iodine are currently widely used in the prevention and management of infection, within the WBP concept. Without WBP, certain patients may be at risk of untreated infection in the wound, which may progress to sepsis (EWMA, 2006).
Topical antiseptic/antimicrobial agents represent the first-line treatment in the management of bacterial burden as they provide a high concentration of the agents at the site of infection (Cutting et al, 2005). Wounds exhibiting signs of increased bacterial burden, wounds that are at risk of bacterial contamination and wounds in immuno compromised patients should be considered for antimicrobial dressings. Topical antiseptic/antimicrobials are recommended as they have a broad spectrum of antimicrobial activity and antimicrobial resistance is rare in human pathogens (Cutting and White, 2004).
The 2-week challenge rule (Wounds UK, 2010) should apply to determine the duration of the use of an antimicrobial dressing. In wounds that are thought to be critically colonised, a topical antimicrobial agent may be considered.
The selection of the appropriate wound management product should be done following the assessment of tissue types present, the level of exudate and patient comfort. Failure of the wound to improve after 2 weeks is an indicator to change to a different dressing and/or add an appropriate antibiotic if signs of infection are progressing (Wounds UK, 2010). The prevention and management of infection and biofilms in chronic wounds is a primary objective. The treatment goal must be to reduce the bioburden and/or eradicate potential biofilm in the wound bed. The incorporation of a method of aggressive wound debridement will help control bacteria biofilms, suppress biofilm regrowth, disrupt the microbial burden and promote wound healing (Sussman et al, 2014; Swanson et al, 2014).
| Table 1. Stages of chronic wound infection continuum | |||
|---|---|---|---|
| Vigilance required | Intervention required | ||
| Not infected / contamination | Colonisation | Critical colonisation / localised infection | Systemic infection |
| The presence of bacteria within the wound without a host reaction | Bacteria are present in the wound and may multiply, but do not initiate a host reaction | Bacteria multiply and delay healing; usually associated with an exacerbation of pain; overt host reaction may be absent (critical colonisation) or present (local infection) | Bacteria multiply, as for critical colonisation/local infection, but also cause a systemic host response, for example, pyrexia, hypothermia or tachycardia |
PHMB
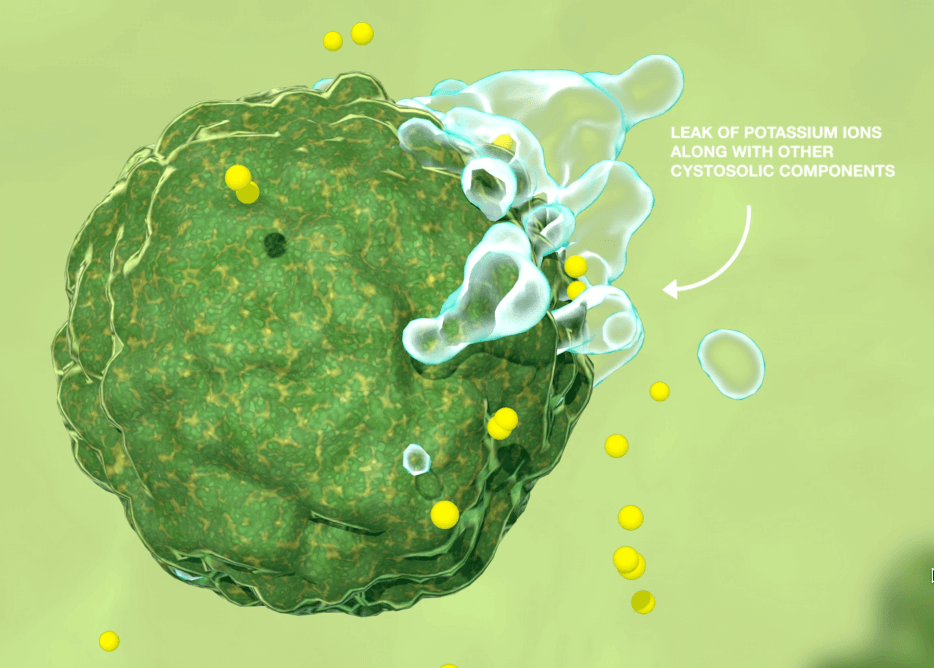
- It adheres and disrupts the target cells’ membrane causing the leak of the components of cytoplasm (Figure 1)
- It can damage or inactivate bacterial DNA (Butcher, 2012).
PHMB has a broad spectrum of activity against bacteria, viruses and fungi, which make it an ideal agent in preventing and managing infection in wound care, where it has previously been identified that diagnosis of pathogens is difficult. The mode of action, which demonstrates the ability to kill the microorganism, supports the observation that there is no evidence of resistance, and the risk of resistance is very low (Butcher, 2012).
PHMB IN WOUND MANAGEMENT
- As a wound irrigation solution
- Impregnated into a carrier dressing, with the intended use of managing the bacterial burden in the wound bed, for example, the ActivHeal PHMB foam range.
Wound care products that incorporate PHMB exhibit the following positive effects on wound healing:
- Good clinical safety
- Target action on bacterial cells
- No known risks of absorption
- No known toxic risks
- Low risk on contact sensitisation
- Sustainability of the active pharmaceutical ingredient
- To assist in the management of reducing the bacterial burden
- Reduce wound pain
- Reduce wound malodour
- Reduce slough and non-viable tissue within the wound
- Increase formation of granulation tissue. (King et al, 2017)
ActivHeal® PHMB foam
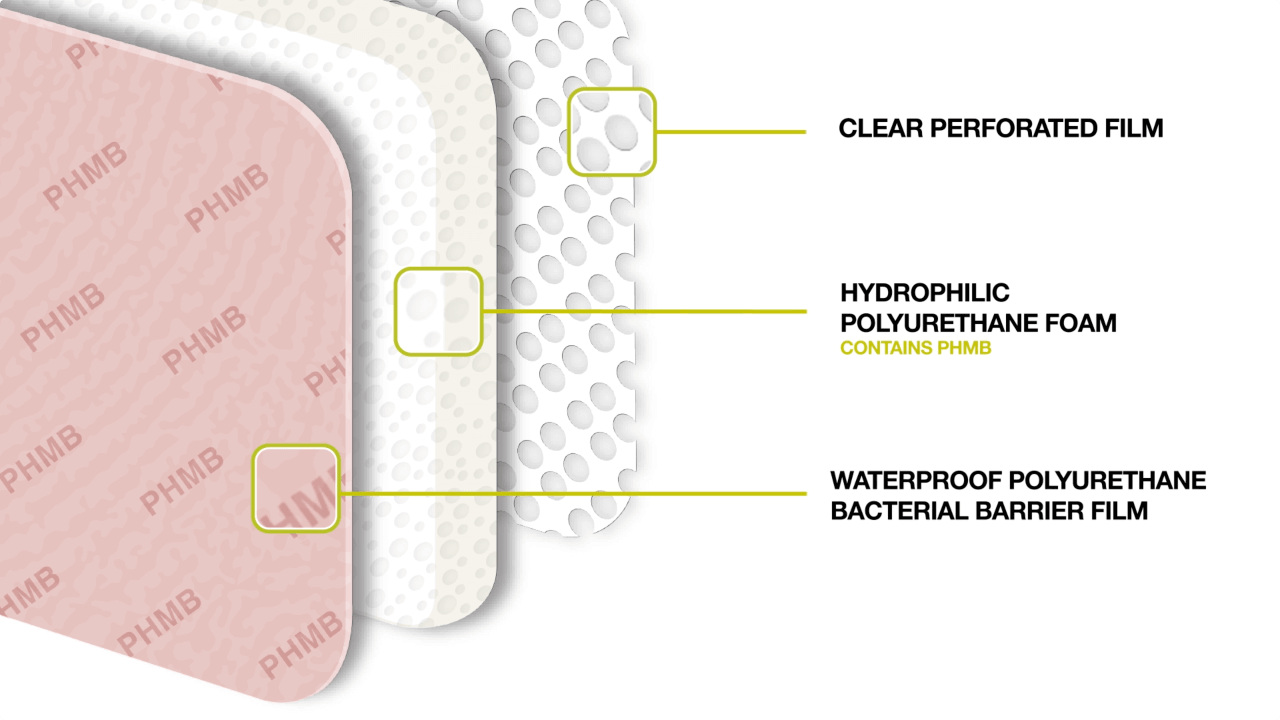
Advanced Medical Solutions Ltd has launched an antimicrobial foam under the ActivHeal range called ActivHeal PHMB foam. The product range includes a non-adhesive and an adhesive bordered range of dressings to address the different clinical needs. The dressing is a three-layer construction, and each layer contributes to the overall performance of the dressing. The wound contact layer prevents adherence of the dressing to the wound bed by preventing the growth of granulating tissue into the dressing, thus reducing trauma to the wound bed upon dressing removal (Figure 2). The polyurethane foam pad contains the antimicrobial substance, PHMB, which is released in the presence of exudate and is effective against a broad spectrum of microorganisms (gram positive, gram negative, fungi and yeasts) that are frequently associated with bacterial colonisation, infection of wounds or wounds at risk of infection. The topmost part of the dressing is a waterproof polyurethane lm, which provides an effective bacterial barrier function, while allowing the transpiration of exudate. The ActivHeal PHMB foam is indicated for use on diabetic ulcers, leg ulcers, pressure ulcers and post-operative.
ACTIVHEAL PHMB FOAM CLINICAL EVALUATION
Methods
The evaluation of the ActivHeal PHMB foam was undertaken over four sites within the UK, to observe the clinical outcome and gain clinician’s opinion of the dressing. The study design was a product evaluation, where the dressing was used within the standard practice delivered by the centre. This was the preferred design to generate information on a range of patients and wound indications, some of which may be excluded in a more controlled study, and to observe current practice when antimicrobial dressings are used.
Within this design, the clinicians were not restricted by a protocol to control the process but were provided with guidelines that included information on the dressing, the inclusion and exclusion criteria and the maximum length of time for the evaluation, which was 4 weeks. Inclusion and exclusion criteria for selection to the evaluation was as follows:
Inclusion criteria:
- Males or females, aged 18 years or above (females must not be pregnant and if of reproductive age should be using contraception)
- Patients who were able to understand and give informed consent to take part in medical treatment
- Patients that had wounds that were infected or at risk of infection.
Exclusion criteria:
- Patients who declined the invitation to take part
- Patients who were known to be non-compliant with medical treatment
- Patients who were known to be sensitive to any of the dressing components.
A copy of the guidelines was attached to the case report form that included a form for the clinician to sign before the patient was included, to con rm the consent of the patient. The patients were not randomised to treatment and no additional interventions were made to standard care, therefore, ethical approval was not required. Organisational consent was obtained along with patient consent.
The ActivHeal PHMB foam was applied to wounds that were assessed as requiring an antimicrobial dressing. These included wounds in which the clinician had assessed infection to be a risk factor, or had observed signs and symptoms that a localised infection may be developing. The primary outcome of the evaluation was to assess the ActivHeal PHMB foam in promoting healing through the management of wound exudate and maintenance of a moist wound environment, as well as controlling the wound bioburden in the management of the following wounds; leg ulcers, diabetic ulcers, pressure ulcers and post- operative surgical wounds. Wound size, wound bed status, exudate levels, peri-wound condition, wound pain, along with the clinical signs and symptoms of infection, were the outcomes measured. The following were evaluated to assess dressing performance: ease of use, conformability to the wound, patient comfort during removal and wear, clinician satisfaction and its ability to stay in place.
Results
The ActivHeal PHMB foam was evaluated on 32 patients. The patients treated were those attending the outpatient services. The age of patients ranged from 46-91 years and wounds were of moderate to high exudate levels. Tissue types were necrotic, sloughy and granulating.
At the end of the evaluation period, 7 wounds had progressed to healing and 14 had progressed significantly to a different wound care product. The other 11 were still receiving the treatment of PHMB foam. No wounds increased in size, and there were positive changes in wound dimensions observed. There was a noted reduction in pain reported by all patients and a trend towards lower levels of exudate was seen in the majority of wounds assessed. These results suggest that the foam dressing effectively managed exudate levels and maintained a moist wound environment.
Clinicians were ‘very satisfied’ and ‘satisfied’ for all performance characteristics assessed. The majority of clinicians rated ‘very satisfied’ and ‘satisfied’ in respect to exudate management, maintaining a moist wound environment, and wound progression. Satisfaction was high with regards to ease of use, with 60% stating they were ‘very satisfied’ and 40% ‘satisfied’ (application and removal). Overall, patients were satisfied with the comfort of the dressing, (88%). Twelve percent stated that they were dissatisfied, mainly due to the adhesiveness of the dressing. Clinical evaluation conclusion The initial results of this clinical evaluation are promising and meets the primary objectives, providing good evidence that the ActivHeal PHMB foam had positive healing outcomes and performance for patients with hard-to-heal wounds including leg ulcers, diabetic ulcers, pressure ulcers and post-operative wounds. The full results will be reported at a later date to further explore the findings. The treatment was well tolerated by the patients overall, and the level of satisfaction for the clinicians in regards to the treatment was high.
CASE STUDY PATIENT 1
Figure 3. Case study 1
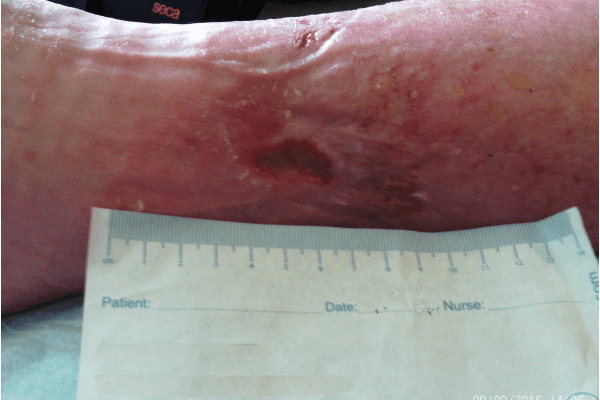
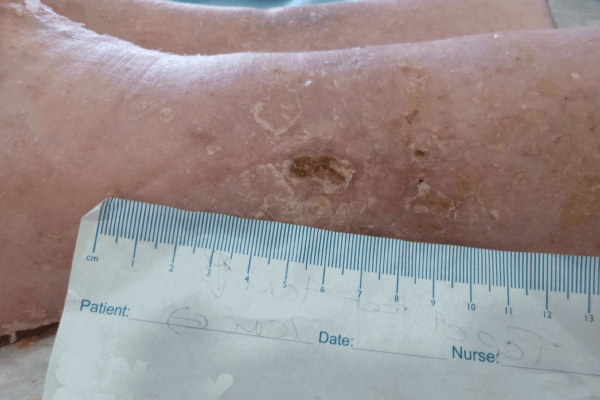
Initial assessment (Figure 3a) found the wound to be 2cm in length and 1.5cm in width, with a high level of exudate. There was also a small wound of less than 1cm. The larger wound had 90% slough and 10% granulation tissue. The wound showed clinical signs of infection and of erythema, increased levels of exudate, pain and purulent discharge. The peri-wound area was also macerated. The patient’s pain score was 8 using a visual analogue scoring scale. ActivHeal PHMB foam non-adhesive was selected to assist in reducing wound bioburden, and absorb and manage levels of exudate, maintain a moist wound environment, facilitate autolytic debridement and promote healing. Compression bandage was also used.
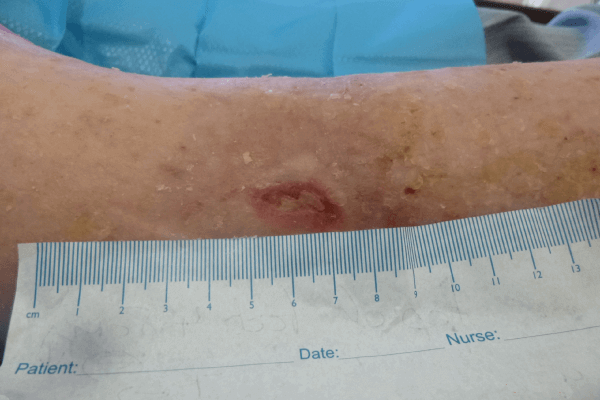
The wound at week 3 (Figure 3b) showed significant progress, with the wound reducing in size and showing wound progression. Areas of slough had reduced and new epithelial tissue was apparent. The wound size was now 1.8cm in length and 0.5cm in width. The wound had a 100% thin layer of sloughy tissue. The peri-wound skin was now intact and no maceration observed, indicating that the ActivHeal PHMB foam non-adhesive was managing the levels of exudate. The patient’s pain score was 4 using a visual analogue scoring scale. The clinical signs and symptoms of infection had reduced, with less erythema and pain; this may be as a result of the reduction in bioburden. The ActivHeal PHMB foam non-adhesive was reapplied as it was effectively managing the clinical symptoms of infection along with exudate levels. As the wound was showing signs of progression and a reduction in clinical signs of infection, the dressing was to be continued for a further 2 weeks (Wounds UK, 2010).
At week 4 (Figure 3c), the wound had reduced in size and exudate levels, and there were no signs of maceration, indicating that ActivHeal PHMB foam non-adhesive effectively managed exudate. The wound also had no evidence of bacterial bioburden and had an improved wound bed with a reduction in wound dimensions and growth of both new epithelial and granulation tissue along with a reduction in pain using a visual analogue scale. ActivHeal PHMB foam non-adhesive was discontinued and the patient moved onto a different therapy.
Case study 1 conclusion
The ActivHeal PHMB foam non-adhesive dressing supported the healing process by reducing the wound bioburden and providing a moist wound environment and aiding autolytic debridement. It offered an alternative approach to wound bioburden and wound management and provided a wound-appropriate method of delivering beneficial effects for both the patient and clinician.
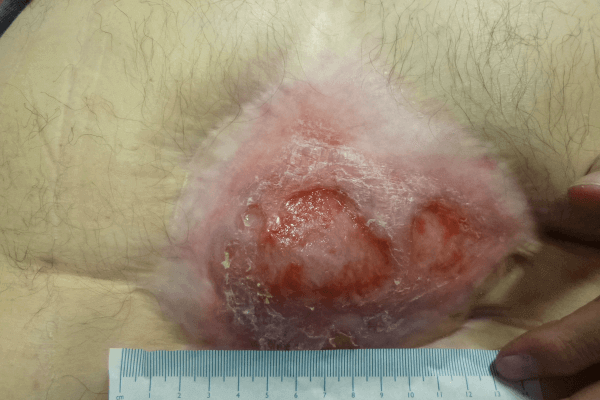
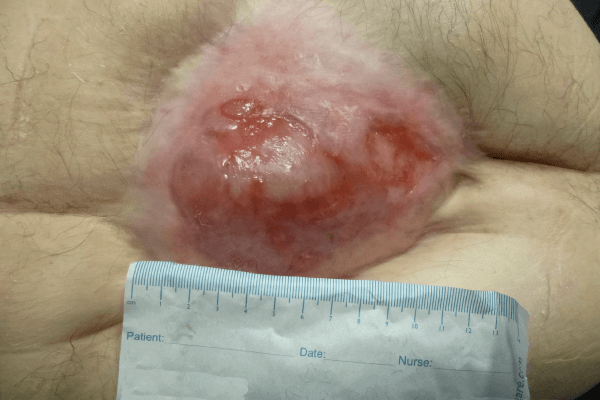
CASE STUDY PATIENT 2
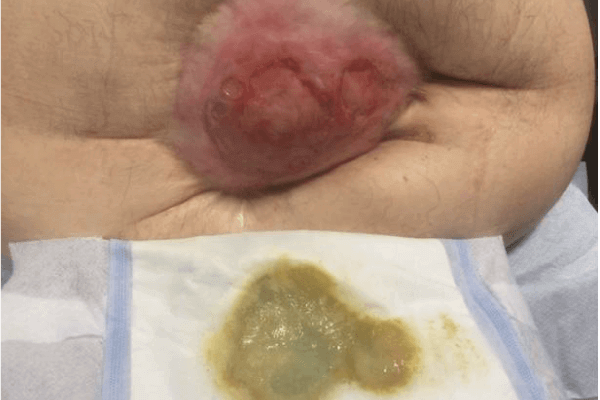
Initial assessment found the wound to be 4cm in length and 4.5cm in width, with moderate exudate, from which there was a purulent discharge. The wound had 40% slough and 60% granulation tissue. The wound showed signs of infection and discolouration of the wound bed, purulent discharge, pain and tenderness and redness/erythema to the surrounding tissue (Figure 4a). The PHMB foam was selected to assist in reducing the wound bioburden and reduce the signs and symptoms of infection as well as to manage and absorb levels of exudate, maintain a moist wound environment, and promote healing.
The wound was reviewed a week later (Figure 4b) and significant progress was then noted in the wound; it was reducing in size and showing positive signs of healing. Areas of slough had reduced and new epithelial tissue was apparent. The peri-wound skin remained intact indicating that the PHMB foam was managing the levels of exudate. The colour of discharge had changed and was less purulent. The clinical signs and symptoms of infection had reduced, with less erythema and pain. The PHMB foam was reapplied.
The wound was reviewed and reassessed at week 3 (Figure 4c) and the wound had reduced further in size and was continuing to heal. Areas of slough had reduced further and healthy granulating tissues was observed along with further areas of new epithelial tissue. The wound was now measuring 3cm long and 4cm in width. Exudate levels were reducing. The peri-wound skin remained intact indicating that the PHMB foam was managing the levels of exudate. No clinical signs of infection were noted. The PHMB foam was discontinued and replaced with a non-antimicrobial foam.
Case study 2 conclusion
ActivHeal PHMB foam dressing was found to be an appropriate dressing producing positive outcomes for the patient with this longstanding wound. The role of the foam as the carrier dressing was relevant, as wounds where the bioburden rises are most likely to demonstrate an increase in exudate. Despite the patient having a complex wound; size, exudate level and excoriation to the surrounding tissue all reduced. The wound also presented no evidence of bacterial bioburden, and the wound bed had improved, with a reduction in wound dimensions and growth of both new epithelial and granulation tissue. Therefore, ActivHeal PHMB foam was not only able to manage the exudate, it also achieved a moist wound environment at the wound bed, along with the removal of harmful bacteria from the wound surface and absorption into the core of the foam dressing. This suggested that ActivHeal PHMB foam can manage bioburden and maintain the moisture balance in the wound bed, addressing two of the barriers to healing within the WBP framework. This case study illustrates the importance of a holistic approach when caring for a patient with a challenging wound and ensuring that the dressing selection is made based upon a thorough assessment, ultimately ensuring good clinical outcomes for the patient.
CONCLUSION
KEY POINTS
- Chronic-wound exudate contains harmful components, including bacteria, which can lead to infection
- PHMB has a broad spectrum of activity against bacteria, viruses and fungi, which make it an ideal agent in preventing and managing infection in wound care
- A clinical evaluation of a PHMB foam dressing demonstrated that it effectively managed exudate levels and maintained a moist wound environment
Declaration of interest: this article was supported by Advanced Medical Solutions Limited
References
- Butcher M (2012) PHMB: an e ective anitmicrobial in wound bioburden management. Br J Nurs 21(12): S16-21. https://doi.org/10.12968/ bjon.2012.21.Sup12.S16
- Cooper RA, Bjarnsholt T, Alhede M (2014) Biofilms in wounds: a review of present knowledge. J Wound Care 23(11): 570–80 https:/doi. org/10.12968/jowc.2014.23.11.570
- Cutting KF,White R (2004) Defined and refined: criteria for identifying wound infection revisited. Br J Community Nurs 9(3 Wound Care Suppl): S6-15. https://doi.org/10.12968/bjcn.2004.9.Sup1.12495
- Cutting K,White RJ, Mahoney P, Harding K (2005) Clinical identification of wound infection: a Delphi approach. In: European Wound Management Association Position Document. Identifying Criteria for Wound Infection. https://tinyurl.com/gobslro (accessed 10 May 2017)
- European Wound Management Association (2006) Association Position Document: Identifying Criteria for Wound Infection. https://tinyurl.com/ gobslro (accessed 10 May 2017)
- Gjodsbolk K, Christensen JJ, Karlsmark T, Jørgensen B, Klein BM, KrogfeltKA (2006) Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3(3): 225-31. https://doi.org/10.1111/ j.1742-481X.2006.00159.x
- Gottrup F, Apelqvist J, Bjarnsholt T et al (2013) EWMA document: Antimicrobials and non-healing wounds. Evidence, controversies and suggestions. J Wound Care 22(5 Suppl): S1-89. https://doi.org/10.12968/ jowc.2013.22.Sup5.S1
- Guest JF, Ayoub N, McIlwraith T et al (2017) Health economic burden that different wound types impose on the UK’s National Health Service. Int Wound J 14(2): 322–30. https://doi.org/10.1111/iwj.12603
- Gray D, Barrett S, Battacharyya M et al (2010) PHMB and its potential contribution to wound management. Wounds UK 6(2): 40-6
- Guo S, DiPietro LA (2010) Factors A ecting Wound Healing. J Dent Res 89(3): 219–29. https://doi.org/10.1177/0022034509359125
- King B, Barrett S, Edwards-Jones V (2017) PHMB made easy. Wounds International, London
- Sibbal G, Orsted H, Schultz G (2003) Preparing the wound bed: Focus on infection and inflammation. Ostomy Wound Manage 49(11): 24-51
- Sorenson O, Cowland J,Theilgaard-Mönch K, Liu L, Ganz T, Borregaard N (2003) Wound healing and expression of anti microbial peptides(polypeptides in human Keratinocytes a consequence of common growth factors. J Immunol 170(11): 5583-9
- Sussman G, Swanson T, Black J et al (2014) Ten top tips: reducing antibiotic resistance. Wounds International 5(4): 4-8. http://tinyurl.com/kp5u285 (accessed 10 May 2017)
- Swanson T, Grothier L, Schultz G (2014) Wound infection made easy. Wounds International. http://tinyurl.com/m7t4mrg (accessed 10 May 2017)
- Welch D, Forder R (2016) The management of a neuropathic diabetic foot ulcer using ActivHeal® PHMB Foam. The Diabetic Foot Journal 19(4): 205-9
- World Union of Wound Healing Societies (2008) Wound infection in clinical practice. An international consensus. MEP ltd, London. http://tinyurl.com/ oglcy8c (accessed 10 May 2017)
- World Union of Wound Healing Societies (2016) Position document: management of biofilm. Wounds International, London Wounds UK (2010) Best practice statement: the use of topical antiseptics/ antimicrobials in wound management.
- Wounds UK, London. http://tinyurl. com/4yxwzf8 (accessed 10 May 2017) Wounds UK (2013) Best Practice Statement:The use of topical antiseptic/ antimicrobial agents in wound management. Wound UK, London. http:// tinyurl.com/lpu5lpw (accessed 11 May 2017)
CONTACT US FOR MORE INFORMATION
DOWNLOADS
ActivHeal® Product Range Catalogue
(PDF – 19 Mb)
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd


