Clinical Resource: Gelling alginate dressings and their contribution to wound management
Carolynne Sinclair and Mandy Yorke
Countess of Chester Hospital and St Mary’s Community Health Campus, Portsmouth
Clinical Resource
Gelling alginate dressings and their contribution to wound management
Carolynne Sinclair and Mandy Yorke
Countess of Chester Hospital and St Mary’s Community Health Campus, Portsmouth
The authors of this article discuss the development of alginate technology and its use within the management of exuding wounds and assess how ActivHeal Aquafiber® performs, and observe wound progression within standard care. A clinical market evaluation was conducted in two UK sites. The primary objective of the study was to observe the wound progression in terms of wound size and condition of the wound bed. The outcome of the evaluation demonstrated Activheal Aquafiber® effectively manages exudate, can assist in autolysis and improves peri-wound status.
BACKGROUND
Posnett and Franks (2008) have calculated that 200,000 people in the UK have a chronic wound, with an estimated treatment cost of between £2.3billion and £3.1billion per year. Chronic wounds have proven costly to the NHS due to prolonged treatment periods, frequent dressing changes, more nursing time used and the potential for further deterioration (Harding et al, 2007). The challenge of chronic wounds remains significant in terms of clinical management, impact on patients and cost to the NHS. Chronic wounds by nature often have clinical features that are challenging to treat and are complicated by the presence of other comorbidities. Chronic wounds may be large in size, have sloughy or necrotic tissue present, be at risk of infection and may have excessive levels of exudate (Vuolo, 2009). The management of wound exudate is one of the key components of an effective wound dressing. How effectively a dressing manages wound exudate affects a number of factors, including condition of the surrounding skin, wear time and healing rates and patient quality of life (World Union of Wound Healing Societies [WUWHS], 2007). The challenge in managing exuding wounds is to maintain a moist wound-dressing interface, while at the same time possibly effectively absorbing and retaining exudate, keeping exudate away from the skin, performing under compression bandaging, being easy to remove, and being cost-effective (White and Cutting, 2006). Fibrous dressings are a popular absorptive dressing that are indicated for wounds with moderate to high levels of exudate. There are two main types of fibrous dressings in wound care: natural fibres and synthetic fibrous dressings. Synthetic fibrous dressings, also commonly known as hydrofiber dressings, are similar to alginate dressings and are indicated for the same wound types.
EXUDATE MANAGEMENT
Wound exudate is a key component of wound healing in a healthy wound. It is produced throughout the healing process from inflammation to epithelialisation and must be managed to maintain a moist wound environment that promotes healing (Collins et al, 2002). Wound exudate can give clinicians many challenges and it is important to achieve and maintain an optimum moist environment. The challenges include:
- Removing harmful bacteria and enzymes from the wound to reduce instances of delayed healing
- Retaining and controlling exudate levels to prevent maceration
- Minimising patient pain and discomfort during dressing changes or when dressing is in situ
- Containing cost while providing effective care (WUWHS, 2007)
Exudate is defined as a fluid produced in wounds, made up of serum, leukocytes and wound debris. The volume of exudate reduces as healing progresses. Exudate is thought to have bacterial and nutrient properties (Adderley, 2008). It facilitates the migration of vital tissue-repairing cells and provides essential growth factors and nutrients for wound healing (White and Cutting, 2006). Exudate facilitates wound bed autolysis of dead or devitalised tissue and transports essential cell metabolising nutrients, growth factors and immune cells as well as preventing the wound drying out (WUWHS, 2007). In non-healing wounds, excessive amounts of exudate can prolong the inflammatory phase, impede growth factors, and prevent or delay cell proliferation (WUWHS, 2007). If wound levels increase and are not effectively managed, the wound bed will become over hydrated, leading to excessive moisture forming on the peri-wound skin and further tissue damage (Tickle, 2012). Poor management of exudate can lead to increased demands on clinicians’ time and resources. Dressing selection should be tailored to the condition of the wound and the peri-wound skin following a full wound assessment. Thomas (2008) identified key characteristics of effective wound dressings that included that the dressing absorbs and retains exudate, keeps harmful exudate away from healthy intact skin, performs under compression therapy, is non-traumatic on removal and is effective in both cost and wear time.
DRESSING DEVELOPMENT AND USE
The term ‘fibre’ dressings is used to describe products manufactured using alginates or carboxymethylcellulose products, which are also known as hydrofibers. These dressing products have similar uses in clinical practice, in that they are used primarily to absorb wound exudate. Once dressings becomes moistened, they retain the exudate, forming a gel product. As such they are able to assist in the debridement of soft slough. Alginates have been used within the wound care industry since the early 1940s and are still considered a complex and versatile dressing, despite newer technologies becoming available (Clarke, 2012). The manufacture of alginates was first reported in the 1800s with the first commercial production in the 1930s (Thomas, 2000a), with alginates being used for a variety of applications. Rinaudo (2014) discusses that alginates have also been used in food for their gelforming ability in jams and jellies along with use in packaging, paper, textiles and the pharmaceutical industry. The use in medical textiles was a growing field, and the use of alginates showed great expansion in wound management products. The first clinical reports were recorded using alginates in 1983; being used for haemostasis, absorption of exudate, absorbability in tissue and lack of toxicity (Fraser and Gilchrist ,1983; Gilchrist and Martin, 1983). The upsurge in the use of alginates in the early 1980s arose through the growing interest in the treatment of acute and chronic wounds (Clarke, 2012).
ALGINATE TECHNOLOGY
Alginates in their natural form are the cell-wall constituents of marine brown algae (Phaeophycea). Alginates are extracted from a variety of species of seaweed, mainly laminaria, Macrocytis and Ascophyllum (McHugh, 1987). Alginic acid is extracted from seaweed and then purified. Alginic acid is a linear polymer with two monomers known as D-mannuronic acid (M) and L-guluronic acid (G) (Draget et al, 2005). Different seaweeds and different parts of the seaweed, i.e. leaves and stem, give rise to varying ratios of the two monomers M and G. The alginic acid is then reacted with sodium chloride to form sodium alginate, and dried to form a powder. Sodium alginate is dissolved in water to form a thick solution. This is forced under pressure through tiny apertures into a solution of a calcium salt. An ion exchange reaction occurs where sodium in the alginate is replaced by calcium. The calcium crosslinks the polymer to make it insoluble and form the fibres (Thomas, 2000b). This is the foundation for alginate wound dressings. The proportions and arrangements of the M, G and MG blocks have an effect on the chemical and physical characteristics of the alginate and any fibre dressing made from it. The higher the content of guluronic acid in the alginate, the greater the interaction and the more stable and harder the gel, therefore giving the alginate wet strength and one-piece removal (Thomas, 2000a). In high M alginates there is an increased fibre swelling and faster gel formation. High M alginates form softer gels than those rich in high G (Thomas, 2000a).
The differences in gel structure caused by the differences in chemical structure have important implications for the products’ clinical use. When the alginate dressing comes into contact with an exuding wound, an ion exchange takes place between the calcium, ions in the dressing and the sodium ions in the wound fluid. When a significant proportion of the calcium ions on the fibres have been replaced by sodium the fibre swells and partially dissolves, forming a gel-like structure (Rinaudo, 2014). In this way they produce moist wound healing conditions that create a moist wound healing environment as well as promoting autolysis (Benbow, 2005). Coagulation is an important part of haemostasis and is an essential part of the healing process for both acute and chronic wounds. Blaine in 1951 demonstrated that alginate dressings were haemostatic. Calcium ions released from the dressing in exchange for sodium ions in the blood activate the clotting cascade by stimulating platelets and clotting factors. In certain clinical situations the absorption of blood by dressings is paramount. Alginates are used to pack or cover the wound to aid in haemostasis, absorb blood or exudate and provide a moist wound healing environment. Alginates can act as a haemostat to control minor bleeding in superficial wounds (Thomas, 2000b). Alginates are also known to break down to simple monosaccharide type residues and be totally absorbed. The wound exudate converts the calcium to the sodium salt, facilitating the removal of the dressing by dissolution. Any residual fibres remaining in the wound are biodegradable thus eliminating the need for complete removal (Barnett and Varley, 1987). Alginates are viewed as being biocompatible, hydrophilic and biodegradable under normal physiological conditions (Becker et al, 2001). Once in a gel form, alginate dressings will also promote healing and epidermal regeneration (Timmons, 2009).
FIBRE DRESSINGS AND ECOLOGY
The use of ‘natural’ products is attractive to a number of individuals, and as such the use of alginates manufactured by Advanced Medical Solutions (AMS) are harvested from renewable sources in Scotland. AMS is one of a few British manufacturers who produce their products in a purpose-built environmentally friendly facility in the UK, with the aim of reducing the impact on the environment. This includes measures to produce lower power usage, emissions and less waste.
ACTIVHEAL AQUAFIBER®
ActivHeal Aquafiber® is a highly absorbent, nonwoven high M gelling alginate fibre dressing with a reinforced hidden web, which is needled into the felt during the manufacturing process. This reinforcement gives the dressing a high wet tensile strength, so that it can be removed intact without leaving any fibres in the wound (Kesteven et al, 2012). When the dressing fibres come into contact with exudate, they swell and form a soft cohesive gel dressing that provides an ideal moist environment to support wound healing. Once in a gel form, alginate dressings will also promote healing and epidermal regeneration (Timmons, 2009). The dressing provides an environment that aids in the facilitation of autolysis of devitalised tissue and managing excess exudate (Hawkins, 2010). It is indicated for use as a primary dressing in exuding acute and chronic wounds; however, a secondary dressing may be required based on the level of exudate. It is designed for use in the management of medium to heavily exuding full thickness, partial thickness, acute and chronic wounds. Aquafiber® is able to absorb a large amount of wound exudate. In vitro testing indicates 23g of fluid per 100 cm2 of dressing over a 24-hour period (AMS, 2013). Aquafiber® is not recommended for use on patients with dry wounds but can be used as a haemostat to control minor bleeding (Thomas, 2000a). Activheal Aquafiber® has the capability to absorb exudate vertically into the dressing, reducing the risk of maceration and damage to the peri-wound skin or to the wound itself (Timmons, 2008; Ousey et al, 2011). ActivHeal Aquafiber® meets the key characteristics of the absorption and retention of exudate, reduced lateral wicking, aiding of autolysis and providing a moist wound healing environment (Ousey et al, 2011). It is vital when selecting dressings to absorb and manage exudate that the product’s components and its mode of action are fully understood in order to ensure correct selection.
| Table 1. Number of patients and range of wound types | |||
| Wound Types | Number of Patients | Wound Diagnosis | Number of Wounds |
|---|---|---|---|
| Pressure ulcers | 6 | Grade 3 Grade 4 |
2 4 |
| Leg ulcers | 4 | Venous Arterial |
4 1 |
| Surgical wounds | 6 | New surgically debrided wound Dehisced/delayed healing |
1 5 |
| Surgical wounds | 4 | Moisture lesion Cellulitis Haematoma Diabetic |
1 1 1 1 |
EVALUATION
An evaluation of ActivHeal Aquafiber® was undertaken in two sites within the UK, to observe the clinical outcome and clinician’s opinion of the dressing. The design was a product evaluation, where the dressing was used within the standard practice delivered by the centre. This was the preferred design to generate information on a wide range of patients, some of which may be excluded in a more controlled study, and to observe current practice when alginate dressings are used. Within this design, the clinicians were not restricted by a protocol to control the process, but were provided with guidelines in which information on the dressing was included, the inclusion and exclusion criteria and the maximum length of time for the evaluation, which was four weeks.
A copy of the guidelines was attached to the data capture documentation that included a form which was signed by the evaluating clinician before the patient was included, to confirm that consent was obtained from the relevant organisation, the patient (which included medical photography for publication) and the patient’s medical practitioner. The primary outcome of the study was to observe the wound progression in terms of wound size and condition of the wound bed. Secondary objectives included the frequency of dressing change, the level of exudate, infection status and peri wound skin condition at the start and end of the evaluation. Once the patient was assessed as suitable for the evaluation and the appropriate consent given and documented, the dressing was applied according to the manufacturer’s instructions for a maximum of four weeks or until the clinician assessed that the dressing was no longer appropriate and an alternative product would be more beneficial or the wound had healed. Patient comfort was also assessed during the evaluation and, if requested by the patient, the dressing would be discontinued.
At the initial assessment, the patient’s age, sex, comorbidities and medication were documented. Patient confidentiality was maintained throughout both in the documentation and wound photographs. Following this specific wound information was recorded to include the aetiology, site, size and the percentage of healthy and unhealthy in the wound bed tissue which was estimated by the clinician undertaking the assessment. A baseline assessment of exudate level, infection status, and condition of the periwound skin was included and an initial photograph taken. These parameters were re-assessed and recorded at each subsequent dressing change. Although ActivHeal Aquafiber® was being evaluated, no other changes to clinical practice were made. The clinicians would clean and debride the wound as planned for each patient within the standard practice, and supporting therapies such as compression bandaging and offloading of the wound would be as required.
However, as this product requires a secondary dressing, this was left to the discretion and clinical judgement of the clinician. All data was recorded on a standardised data capture form by the clinicians who treated the patient and at the end of the evaluation process was analysed using a simple Excel spreadsheet. Because of the small numbers of patients and study design, statistical analysis was not planned, although the outcomes of the evaluation may be used to power larger, more controlled comparative studies.
OUTCOMES
The evaluation was undertaken in two different environments — a ward environment within an acute hospital, and a podiatry clinic, which treated complex foot wounds on an outpatient basis. As a result, the wounds varied in aetiology, size and duration; however, they were all assessed as requiring an alginate primary dressing at the wound bed to facilitate the management of excess exudate. The evaluation took place over a 6-month period, where ActivHeal Aquafiber® was used on 20 patients (5 males and 15 females) with ages ranging from 43 to 88 years with a mean of 72.3 years. Table 1 demonstrates the range of wound aetiologies included. The primary objective of the product evaluation was to observe wound progression when using ActivHeal Aquafiber® within standard care. This was determined by initially measuring the size of the wound by clinicians using a sterile ruler to measure the maximal length by the maximal perpendicular width (Gethin, 2006). Deep cavities were probed to identify the full extent of tissue damage. There was a wide range of wound sizes and depths included in the evaluation.
ACTIVHEAL AQUAFIBER®
ActivHeal Aquafiber® is a highly absorbent, nonwoven high M gelling alginate fibre dressing with a reinforced hidden web, which is needled into the felt during the manufacturing process. This reinforcement gives the dressing a high wet tensile strength, so that it can be removed intact without leaving any fibres in the wound (Kesteven et al, 2012). When the dressing fibres come into contact with exudate, they swell and form a soft cohesive gel dressing that provides an ideal moist environment to support wound healing. Once in a gel form, alginate dressings will also promote healing and epidermal regeneration (Timmons, 2009). The dressing provides an environment that aids in the facilitation of autolysis of devitalised tissue and managing excess exudate (Hawkins, 2010). It is indicated for use as a primary dressing in exuding acute and chronic wounds; however, a secondary dressing may be required based on the level of exudate. It is designed for use in the management of medium to heavily exuding full thickness, partial thickness, acute and chronic wounds. Aquafiber® is able to absorb a large amount of wound exudate. In vitro testing indicates 23 g of fluid per 100 cm2 of dressing over a 24-hour period (AMS, 2013). Aquafiber® is not recommended for use on patients with dry wounds but can be used as a haemostat to control minor bleeding (Thomas, 2000a). Activheal Aquafiber® has the capability to absorb exudate vertically into the dressing, reducing the risk of maceration and damage to the peri-wound skin or to the wound itself (Timmons, 2008; Ousey et al, 2011). ActivHeal Aquafiber® meets the key characteristics of the absorption and retention of exudate, reduced lateral wicking, aiding of autolysis and providing a moist wound healing environment (Ousey et al, 2011). It is vital when selecting dressings to absorb and manage exudate that the product’s components and its mode of action are fully understood in order to ensure correct selection.
PRESSURE ULCER WOUNDS
- 30% (n=6) of patients presented with pressure ulcers (1 of which was grade 3, the remaining were grade 4). These ranged from 32 to 300 cm2, with a mean of 110.6 cm2. These wounds had cavities that required packing, one of which was extensive and extended down to bone, and one of which undermined by 18 cm. At the end of the evaluation all wounds had improved although the mean size was still 81.8 cm2, but only three patients had cavities that required packing, the maximum depth of which was 7 cm.
- Initially all of these patients were assessed as having high levels of exudate, but at the end of the 4-week evaluation period this was considered to be ‘moderate’ by the clinician in four patients, and the frequency of dressing change was reduced to alternate days or every 3 days.
- The secondary dressing used in conjunction with ActivHeal Aquafiber® was a foam adhesive dressing (20% of patients, n=4) and an absorbent pad (10% of patients, n=2).
- 25% of patients (n=5) initially had excoriated or inflamed skin in the periwound area. At the end of the evaluation period they were all assessed as having healthy tissue present.
LEG ULCERS
- Four patients with leg ulcers were treated in the evaluation, which was 20% of the total number. The wound area was similar in these patients, ranging from 118 cm2 to 122 cm2, with a mean of 120 cm2. A patient who presented with a large arterial wound was also documented as having bone and tendon exposed. At the end of the evaluation period this had been covered with granulation tissue, and the mean wound size of all wounds had reduced to 67.5 cm2.
- All of the leg ulcers included in the evaluation were initially exuding high amounts of exudate. This decreased in two patients by the end of the evaluation period, with data missing for the remaining one.
- Again either a foam adhesive or adhesive pad was used as a secondary dressing. The data evaluation form did not indicate whether compression therapy was also used on the patients with venous disease.
- Three patients were identified to have periwound skin damage at the start of the evaluation, which, resolved by week 2.
SURGICAL WOUNDS
- Two patients in this cohort were treated by a specialist podiatry service, as they were digital amputations in diabetic patients. The remaining four patients were in patients in an acute care setting.
- The wound sizes ranged from 2.8 cm2 to 270 cm2, with five of the wounds presenting with a cavity that required packing. The depth of the cavities varied from 1.4 cm to 15 cm. One patient healed and wound size reduction was recorded in the three remaining patients. The wound size increased in the two diabetic patients, but this may have been attributed to the radical debridement of the wound margins to remove callus.
- Overall the mean wound size reduced from 473.6 cm2 to 50.5 cm2, with a reduction in cavity depth observed in all patients.
- Five patients were initially assessed as having high levels of exudate, with the remaining being moderate. At the end of the 4-week evaluation period, two patients no longer required the alginate dressing as the exudate level was too low, and the remaining two patients were considered to have moderate amounts.
- The wounds of the four patients treated within the acute hospital, were treated with a foam secondary dressing. Those patients treated by the specialist podiatry team had a secondary dressing of sterile gauze. This was used to minimise bulk in the specialist footwear that was required for offloading the wound.
OTHER WOUND TYPES
These included a diabetic foot ulcer, a haematoma, a circumferential cellulitis of the leg, which was weeping copious amounts of fluid and a small moisture lesion on the buttocks.
- All of these wounds were assessed as having high levels of exudate, and therefore were suitable for an alginate dressing. In all four patients a non adherent pad or gauze dressing was applied. This was because of the risk of faecal contamination (moisture lesion) or the wound required daily observation as a result of the presence or risk of infection.
- Only the peri-wound skin of the cellulitic leg was recorded as damaged through excoriation, and this only improved slightly over the evaluation. In the remaining patients, the skin remained intact.
- The wound size of the cellulitic leg was not measured, although the sizes of the remaining wounds ranged from 2 cm2 to 20 cm2. At the end of the evaluation period, the moisture lesion had healed, the haematoma wound had reduced in size, but again there was increase in the wound margins of the diabetic foot ulcer, but extensive debridement had been part of the treatment.
The condition of the wound bed was also observed, with reduction in devitalised tissue indicating a progression towards healing. Although alginate dressings are not always the first choice for debriding wounds, the gelling action of the product can assist in autolysis, which can be an additional benefit to managing excess exudate. This was demonstrated in Figure 1 where a decrease in viable tissue was observed. At the start of the evaluation, 45% (n=9) patients were recorded as having 100% of non-viable tissue which reduced to 5% (n=1) at the end. In addition, 40% of patients (n=8) were recorded as having 25% or less viable tissue in the wound bed, which then increased to 95% (n=19) at the end of the evaluation. This suggests that ActivHeal Aquafiber® can provide an environment to support wound progression by not only managing exudate balance but also providing a maintenance debridement function.
The secondary objectives of the study were to observe the effectiveness of ActivHeal Aquafiber® in managing wound exudate. Alginate dressings are indicated for moderate to highly exuding wounds, and because of their ability to absorb up to 20 times their own weight in fluid can reduce the frequency of dressing change (Thomas, 2000b). Excess wound exudate that leaks on to the peri-wound skin can cause excoriation and maceration, which can be uncomfortable for the patient and promote further wound deterioration.
The outcome of the evaluation demonstrated that:
- 90% of patients (n=18) were recorded to have high levels of exudate at the start. At the end of the evaluation period the exudate levels had reduced in 90% of patients (n=18), with the final data missing for 1 patient (Figure 2). Clinicians had been asked to assess the exudate level using the preset criteria of low, moderate and high — and while this may be subjective and inconsistent, this is reflected in how it is measured in everyday practice.
- Only 25% of patients (n=5) were initially observed to have healthy tissue surrounding the wound, indicating that the previous dressing regimen was not protecting periwound skin from excoriation or maceration. An improvement in the peri-wound skin was observed at the end of the evaluation period when this increased to 90% (n=18) after the plan of care was changed to include ActivHeal Aquafiber®
- 35% (n=7) of patients were identified to have a wound infection at the initial assessment, all of which were treated with systemic antibiotics. While ActivHeal Aquafiber® is not an antimicrobial dressing, daily dressing changes were undertaken and no new infections developed in the evaluation wounds.
- The choice of secondary product was either a foam or non woven dressing. As a product evaluation, the choice of secondary dressing was left to the discretion of the clinician, and as such may have contributed to the outcome of the evaluation.
- All clinicians reported that the dressing was easy to use, and was conformable to the wound bed.
DISCUSSION
CONCLUSION
CASE STUDY
Treating complications of injection anthrax using ActivHeal Aquafiber® and ActivHeal Foam Adhesive®
Patient A is a 32-year-old male intravenous drug user who had injected heroin into his right femoral artery. Unknown to the patient the heroin had been mixed with a substance that contained anthrax spores. The patient presented with an area of blistering, extensive bruising and oedema to the right groin (Figure 3) and also bruising to the right anterior hip (Figure 4) due to tissue destruction. The patient was admitted to ICU with multiple organ failure and sepsis. He presented with gross oedema of the right groin, with skin blistering and areas of necrotic tissue. Initially, it was suspected that the patient was suffering from necrotising fasciitis. However, the diagnosis of Bacillus anthracis was confirmed from blood cultures. The patient underwent exploratory surgery to ascertain the depth of the tissue destruction, which resulted in the surgical debridement of the two necrotic areas to viable tissue, which was then referred to tissue viability for further treatment. Following holistic wound assessment from tissue viability, it was decided that, due to the size of the wound and the complications of gross oedema of the trunk and lower limbs, the larger wound would be managed with topical negative pressure due to the size of the wound and the amount of exudate being produced by the wound. With the smaller wound being treated with ActivHeal Aquafiber® and ActivHeal Foam Adhesive® as a secondary dressing.
Treatment aim:
- Exudate management
- Creation of a moist wound healing environment
- Promotion of granulation
- Protection of peri wound skin from maceration due to excessive exudate production.
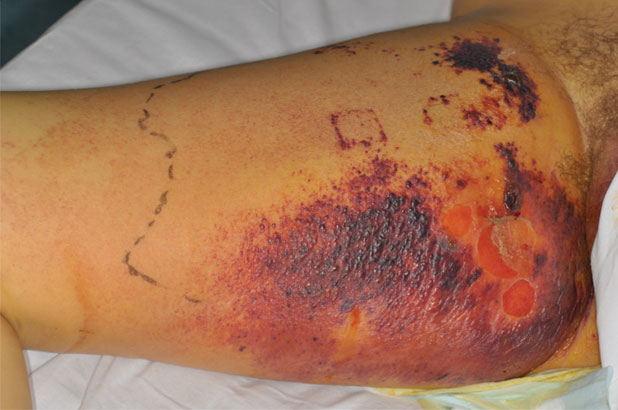
Figure 3. Right groin. Initial assessment (Day 1).
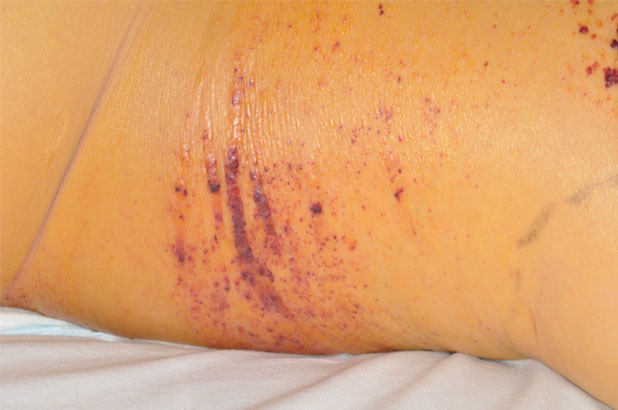
Figure 4. Right lateral hip. Initial assessment (Day 1).

Figure 5. Right lateral hip after surgical debridement (Day 1).
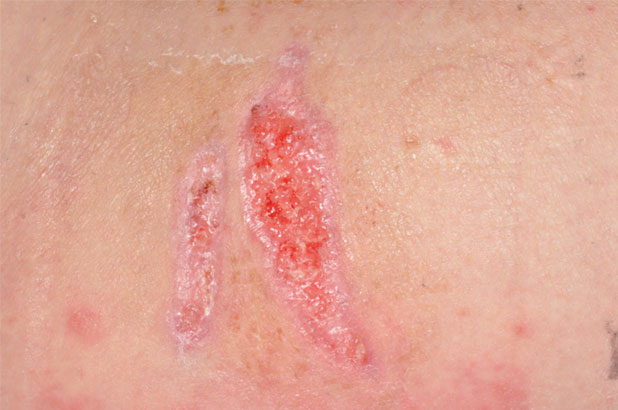
Figure 6. Right lateral hip (Day 27).
MANAGEMENT
DISCUSSION
CONCLUSION
References
Advanced Medical Solutions (2013) Technical Report Activheal Aquafiber. Data on File. Advanced Medical Solutions, Winsford
Adderley U (2008) Wound exudate: What it is and how to manage it. Wound Essentials 3: 8–13
Barnett S, Varley S (1987) The effects of calcium alginate on wound healing. Ann R Coll Surg Engl 69(4): 153–5
Becker TA, Kipke DR, Brandon TA (2001) Calcium alginate gel: a biocompatible and mechanically stable polymer for endovascular embolisation. J Biomed Mater Res 54(1): 76–86
Benbow M (2005) Evidence-Based Wound Management. Whurr Publishers, London
Blaine G (1951) A comparative evaluation of absorbable haemostatics. Postgrad Med J 27(314): 613–20
Clarke M (2012) Technology Update: Rediscovering alginate dressings Wounds International 3(2): 24–8
Collins F, Hampton S, White R (2002) A-Z dictionary of Wound Care. Quay Books, London
Draget KI, Smidsrød O, Skjåk-BrækG (2005) Alginates from Algae. Available at: http://www.wiley-vch.de/books/sample/3527313451_ c01.pdf (accessed 24.06.2014)
Ebright JR, Pieper B (2002) Skin and soft tissue infections in injection drug users. Infect Dis Clin North Am 16(3):697-712
Fraser R, Gilchrist T (1983) Sorbsan calcium alginate fibre dressing in footcare. Biomaterials 4(3) 222–4
Gethin G (2006) The importance of continuous wound measuring. Wounds UK 2( 2): 60–8
Gilchrist T, Martin AM (1983) Wound treatment with Sorbsan – an alginate fibre dressing. Biomaterials 4 (4): 317–20
Grunow R, Klee SR, Beyer W et al (2013) Anthrax among heroin users in Europe possibly caused by same Bacillus anthracis strain since 2000 Euro Surveillance 18(13): 20437
Harding K, Gray DG, Timmons JP, Hurd T (2007) Evolution or Revolution? Adapting to complexity in Wound Management. International Wound Journal 4 (Suppl 2): 1–12
Hawkins E (2010) Using ActivHeal in a traffic light system wound care formulary. Wounds UK 6(4): 177–82
Hendricks KA, Wright ME, Shadomy SV, Bradley JS, Morrow MG, Pavia AT et al (2014) Centers for disease control and prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis 20(2): e130687
Kesteven D, Hinde H, Forder R (2012) Performance characteristics of a reinforced high gelling moist wound dressing. Presented at: Wounds UK , Harrogate, November
McHugh DJ (1987) Production and utilisation of products from commercial seaweeds. FAO Fisheries Technical Paper (288): 147–89
Morris C (2006) Wound Management and dressing selection. Wound Essentials 1(1): 178–83
Ousey K, Edwards C, Jordan J, Sinclair C, Hawkins E, Caddy M (2011)This case series highlights the clinical effectiveness of the Activheal wound care dressing range. BJN ActivHeal Supplement
Posnett J, Franks PJ (2008) The Burden of chronic wounds in the UK. Nursing Times 104(3): 4–5
Rinaudo M (2014) Biomaterials based on a natural polysaccharide: alginate. RevistaEspecializada en CienciasQuimico-Biologicas 17(1): 92–6
Thomas S (2008) The role of dressings in the treatment of moist related skin damage. http://tinyuk.com/65d25x
Thomas S (2000a) Alginate dressings in surgery and wound management – Part 1. J Wound Care 9(2): 56–60
Thomas S (2000b )Alginate dressings in surgery and wound management – Part 3. J Wound Care Vol 9, (3) 163-166
Tickle J (2012) Effective Management of exudate with Aquacel extra. Br J Community Nurs. 17(9 Suppl):S38, S40–6
Timmons J (2008) ActivHeal Aquafiber a new soft, conformable highly absorbent dressings for use with chronic wounds. Wounds UK 3( 3): 2–4
Wick G (2012) Meeting CQUIN Targets: Effective Dressing Selection. Available at: http://www.wounds-uk.com/how-to-guides/meetingcquin-targets-effective-dressing-selection&print (accessed 24.06.2015)
White R, Cutting KF (2006) Modern Exudate Management: A Review of Wound Treatment. Available at: http://www.worldwidewounds. com/2006/september/White/Modern-Exudate-Mgt.html (accessed 24.06.2015)
World Union of Wound Healing Societies (2007) Principles of Best Practice: Wound Exudate and the role of Dressings. A Consensus Document. Available at: http://www.woundsinternational.com/media/issues/82/ files/content_42.pdf (accessed 24.06.2015)
Wounds International (2012) International Consensus: Optimising Wellbeing in People Living with a Wound. An Expert Working Group Review. Available at www.woundsinternational.com/pdf/ content_10309.pdf (accessed 24.06.2015)
Wounds UK (2013) Wounds UK. Best Practice Statement. Effective Exudate Management. London. Wounds UK.2013
CONTACT US FOR MORE INFORMATION
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd


