Clinical Resource: Post-market clinical evaluation of the safety and performance of ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite dressings
Rebecca Forder, Rachel Burns
Advanced Medical Solutions Ltd. UK
Clinical Resource:
Evaluation of ActivHeal® Aquafiber™ Extra dressings performance and acceptability in clinical practice
KEY WORDS
- ACTIVHEAL® AQUAFIBER™ EXTRA DRESSING
- EXUDATE
- HEALING
- OUTCOMES
- PERFORMANCE
BACKGROUND
This post-market evaluation assessed the performance of ActivHeal® Aquafiber™ Extra dressing and gathered feedback from clinicians using it to treat a variety of wounds.
The primary outcomes were the progression to healing through exudate management and maintenance of a moist wound healing environment. The evaluation took place across three sites in the UK.
Forty-three patients were treated according to instructions to use and standard local practice. Data was collected at every dressing change. Aquafiber® Extra use had positive effects on exudate levels, periwound condition and tissue type(s) within the wound bed. The majority of wounds reduced in size during the evaluation period and three completely healed.
Overall, clinician and patient satisfaction were high. No adverse events were reported. The results suggest Aquafiber® Extra dressing use in clinical practice is safe, effective and acceptable to practitioners and patients.
OVERVIEW
The cost of wound care continues to increase year-on-year. The NHS spent an estimated £5.3 billion on the management of wounds and related comorbidities in 2012/13 (Guest et al, 2017). It costs on average 135% more to treat chronic than acute wounds; therefore emphasis should be on identifying the reason for delayed healing, wound prevention and improving healing rates (Guest et al, 2017).
Exudate can hinder chronic wound healing as it: slows and may prevent cell proliferation; interferes with growth factor availability; and contains elevated levels of inflammatory mediators and activated matrix metalloproteinases (MMPs) that break down tissue (Mudge and Orsted, 2010; Romanelli et al, 2010). Effective management of exudate is a fundamental part of wound care.
Patients should receive a treatment plan to ensure a moist wound environment is maintained, the volume of exudate is managed with suitable dressings, the periwound skin is protected, and the cause of the wound or related factors are managed. This should reduce the healing time and subsequently improve the patients’ quality of life (Gardiner, 2012; World Union of Wound Healing Societies [WUWHS], 2019).
Excess exudate is a potential risk factor for infection (Adderley, 2008; Chadwick and McCardle, 2015). The presence of infection and devitalised tissue can result in malodour. Ineffectual handling of exudate can have a detrimental effect on both the wound and the patient — resulting in strikethrough, embarrassment, and damage to the surrounding skin, causing pain and discomfort. The practical, economic and social consequences for the patient can manifest as isolation from friends and family, the need to wash clothes more often and the use of cumbersome dressings that restrict movement and choice of clothing (Dowsett, 2012). It can be difficult to know which dressing to use to manage high levels of exudate. The ideal properties of such dressings include:
- High Absorbency
- Prevention of leakage between dressing changes
- Prevention of strikethrough
- Protection from excoriation/maceration
- Suitable for use under compression
- Stays intact and can be left in place for a long period of time
- Minimisation of trauma and pain on removal
- Comfortable and conformable
- Cost-effective (Adderley, 2008; Stephen-Haynes, 2011; Gardiner, 2012; WUWHS, 2019).
Clinicians should know or obtain information about the fluid-handling, safety, clinical and costeffectiveness of a range of dressings (National Institute for Health and Care Excellence [NICE], 2016). They need to combine an understanding of the fluid-handling characteristics of the dressing with clinical experience when selecting the most appropriate treatment for each patient. It is wise, when choosing a higher-cost product, to ensure that it is cost-effective, as newer technologies can be more expensive.
Selecting a product that will create an optimal environment for wound healing and manage the level of exudate, taking into consideration treatment efficacy, structural integrity and wear time, is key (Naude and Ivins, 2017). Wear time is an important factor, as the number of dressing changes has an impact on community nursing visits, treatment costs and the patient (Dowsett, 2015; WUWHS, 2019). Leaving the correct dressing undisturbed for a longer period of time assists healing (Rippon et al, 2012); when choosing a dressing, the practitioner should aim to reduce the frequency of changes to avoid disrupting the wound healing environment (McGuinness et al, 2004). Selecting a dressing that will effectively absorb and retain exudate can potentially achieve this (Jones et al, 2017).
Patient and/or carer involvement should be considered when choosing a dressing, especially with the move towards self-care and patient empowerment. Patient and/or carer involvement can lead to improved concordance, an understanding of wound progression and an ability to make informed decisions relating to the wound management plan (International Best Practice Statement, 2016). For many patients, the ideal wound dressing is one that is not bulky, reduces the likelihood of pain during dressing change, needs fewer changes and is easy to apply and remove (Dowsett, 2012). Holistic assessment is vital to ensure the correct dressing is selected. This will facilitate faster healing, improve patient outcomes and quality of life (Nazarko, 2018; NICE, 2016).
Some dressing materials have the potential to alter the composition of exudate in ways that may prove beneficial to healing, therefore, possible interactions between the dressing and wound should be considered during the selection process.
Alginates
Alginate dressings have been in use for 2 decades and are primarily used to manage wound exudate. Alginate products are biocompatible, hydrophilic and biodegradable under normal physiological conditions. They form a soft gel on contact with exudate, providing a moist environment at the wound bed that promotes healing and epidermal regeneration (Timmons, 2009; Sood et al, 2014; Aderibigbe and Buyana, 2018).
Alginates have an inherent haemostatic effect and can be used to stop minor bleeding complications, for example after a wound biopsy (Naik and Harding, 2019). Alginate dressings contain alginic acid, usually derived from seaweed; the fibres contain mannuronic and guluronic acid and it is the combination (%) used which gives them the high tensile strength when wet (Naik and Harding, 2019).
Gelling Fibres
Fibre-based dressings are frequently selected due to their ease of use, effective exudate management and relatively low cost. Gelling fibres are structurally similar to alginates. Products containing gelling fibres are comfortable, easy to remove, and can be used with heavily exudating wounds. Together with their absorptive properties, gelling fibres promote tissue granulation and the maintenance of a moist wound environment (NHS Business Service Authority, 2018). They effectively remove MMPs and reduce bioburden due to their highly absorptive nature (Sood et al, 2012).
Cellulose is the most copious naturally occurring fibre with a high tensile strength (Waring and Parsons, 2001; Naik and Harding, 2019). It can be structurally altered to form carboxymethyl cellulose (CMC) on contact with fluids such as exudate. This characteristic helps CMC trap the fluid and components of exudate (e.g. MMPs), along with inflammatory cells, bacteria and debris, when used in gelling fibre dressings, locking them into the fibres and promoting autolytic debridement (Romanelli et al, 2010; Aderibigbe and Buyana, 2018; Naik and Harding, 2019). It is due to these properties that CMC-based dressings are widely used in clinical practice.
RESULTS
Figure 1. Absorbency of dressings containing CMC
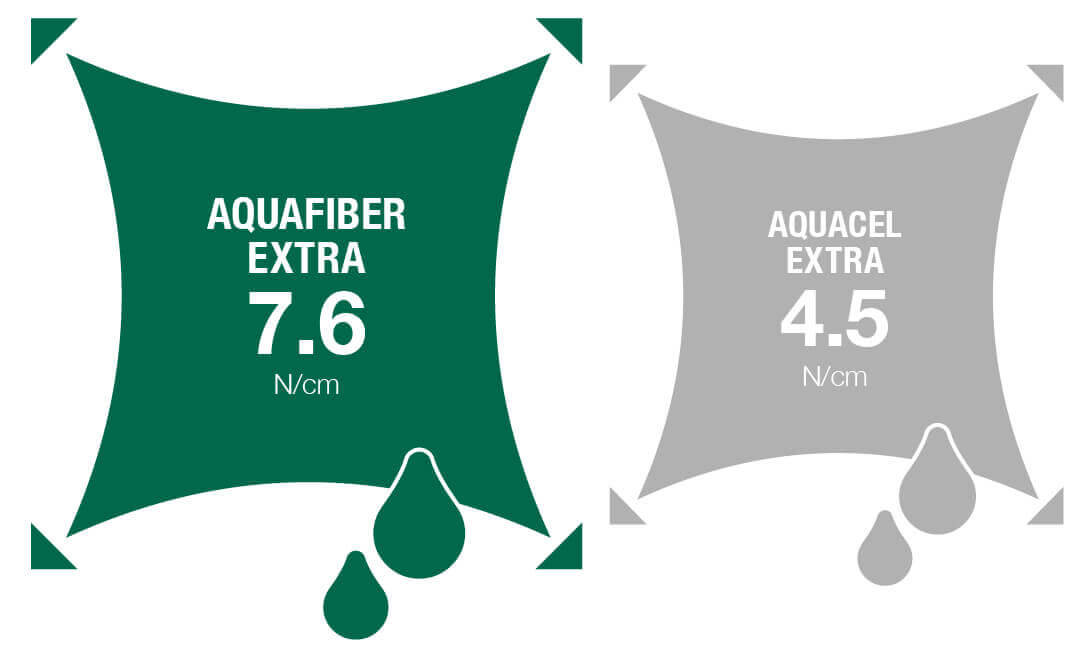
Figure 2. Wet strength of dressings
Figure 3. Dry strength of dressings
Figure 4. Lateral wicking of dressings (cm2)
ACTIVHEAL® AQUAFIBER™ EXTRA
ActivHeal® Aquafiber™ Extra dressing is an innovative combination of two proven wound care technologies: alginate fibres (guluronic acid) and CMC. It is a non-woven, highly absorbent dressing that supports autolytic debridement while helping maintain a moist wound bed in exuding wounds. The dressing effectively addresses the key challenges of improved exudate management, gelling and tensile strength. The fibres turn into a highly absorbent soft cohesive gel on contact with exudate. The level of gelling is designed to maximise the conformability of the dressing to the contours of the wound and ease dressing removal. Its unique patented technology, in conjunction with a hidden integrity layer, increases its tensile strength when in contact with exudate, facilitating dressing removal.
A comparative in-vitro assessment (Walsh et al 2017) showed that Aquafiber® Extra has an absorbent capacity that is 54% higher than a 100% CMC dressing and 31% higher than reinforced CMC dressing (which uses reinforcing threads), see Figure 1. It had greater wet and dry strength than the other dressings tested, see Figure 2 and Figure 3. Aquafiber® Extra was 96% stronger than 100% CMC dressing and 12% stronger than reinforced CMC dressing when wet. When dry, it was 91% stronger than 100% CMC dressing and 22% stronger than reinforced CMC dressing, see Figure 3. Aquafiber® Extra is therefore more likely to remain intact on application and removal. It maintained integrity when wet for a period of 8 days (Walsh et al, 2017 data on file) and will therefore remain intact for the intended wear time.
Dressings with good lateral wicking capture fluid at the point of contact and prevent it from spreading within the dressing. A lower value indicates a better outcome and a lower risk of maceration. Aquafiber® Extra had 24% less lateral wicking than the 100% CMC dressing and 4% less than the reinforced CMC dressing (Walsh et al, 2017 data on file), see Figure 4. These findings demonstrate that ActivHeal® Aquafiber™ Extra is highly absorbent, making it suitable for use on moderately to heavily exuding wounds.
Figure 5. Wound status at the end of the evaluation based on mean wound size
Figure 6. Exudate levels at the beginning and end of the evaluation period
CLINICAL EVALUATION
The primary objective of the clinical evaluation was to assess the clinical safety and performance of Aquafiber® Extra in the management of acute and chronic wounds.
Method
Three sites in the UK participated in this product evaluation. Clinicians were provided with guidelines on the use of Aquafiber® Extra rather than being given a protocol to follow. The dressings were applied, when appropriate, in accordance with each site’s standard practice. Patients were followed up for a maximum of 6 weeks. Information was gathered on a range of patients and wounds. Clinical outcomes were observed and clinicians’ opinions of the dressing gathered.
Patients aged 18 or over and able to understand and give informed consent were eligible to participate. Patients were excluded if:
- They did not wish to take part
- They were women of childbearing age and were pregnant or not using contraception
- They were known to be non-concordant with medical treatment
- They were sensitive to any of the dressing components
Clinicians were provided with a copy of the guidelines along with case report forms and patient consent forms. Patients were enrolled in the evaluation once they had provided informed consent. Participants were not randomised to treatment and no additional interventions were made to standard care; therefore, no ethical approval was required. Organisational consent was obtained for this study. ActivHeal® Aquafiber™ Extra was applied to wounds (leg ulcers, diabetic ulcers, pressure ulcers, post-operative surgical wounds and cavity wounds) that required debridement and a moist wound healing environment.
The primary outcomes of the evaluation were the progression to healing through exudate management and the maintenance of a moist wound environment. Wound size, tissue type(s) present in the wound bed, exudate levels, periwound condition and discomfort levels were measured. Ease of use, conformability to the wound, patient comfort during wear and removal, clinician satisfaction and the ability of Aquafiber® Extra to stay in place were evaluated in order to assess dressing performance.
RESULTS
ActivHeal® Aquafiber™ Extra was used to treat 43 patients aged 36–94 years (average 68 years) attending outpatient services, including individuals at a long-stay rehabilitation hospital. The wounds treated had moderate to high exudate levels and contained necrotic, sloughy and/or granulating tissue.
Clinical efficacy
Figure 5 shows the status of wounds at the end of the study. Reductions in wound dimensions were observed. Initially, mean wound length, width and depth were 3.67 cm, 2.49 cm and 0.56 cm, respectively; at the end of the evaluation the mean length, width and depth were 2.96 cm, 2.16 cm and 0.43 cm. At the end of the evaluation period, 81% (n=35) of the wounds had improved: three had healed and 32 had progressed significantly towards healing and treatment had been stepped down to a different wound care product. Lower levels of exudate were seen in the majority of wounds assessed, see Figure 6.
At the end of the evaluation period, the types of tissue present in the wound bed showed a trend towards healing, see Figure 7. Fourteen per cent (n=6) of wounds showed no progression. Of the six wounds that did not progress to healing, four were diabetic foot ulcers in patients with multiple comorbidities and the remaining wound was a category 4 pressure ulcer in an individual with multiple comorbidities and double incontinence. Antibiotics were prescribed in these cases as the wounds had underlying infection and the local infection protocol was followed. Although wound size remained the same for these wounds, there was a reduction in the percentage of necrotic and sloughy tissue in the wound bed.
User Opinion
Table 1 gives levels of satisfaction with the various characteristics of Aquafiber® Extra assessed. The majority of clinicians were satisfied or very satisfied with the product characteristics. (The exception being wound progression where 14% (n=6) were dissatisfied.) Satisfaction levels were highest for exudate management, ease of use, conformability and patient comfort. One clinician (2%) did not give an answer with regard to the prevention/minimisation of trauma with Aquafiber® Extra. Overall, levels of satisfaction were predominately high.
Safety
There were no safety or adverse events reported during the evaluation. This suggests that for this particular evaluation there are no safety concerns with the use of ActivHeal® Aquafiber™ Extra dressing.
| Table 1. Clinician satisfaction with characteristics of Aquafiber® Extra | ||||
|---|---|---|---|---|
| CHARACTERISTIC | PROPORTION OF PARTICIPANTS (%) | |||
| Very Satisfied | Satisfied | Not Satisfied | No Answer Given | |
| Management of Exudate | 63 | 37 | 0 | 0 |
| Wound Progression | 42 | 44 | 14 | 0 |
| Promotion and facilitation of autolytic debridement | 37 | 67 | 0 | 0 |
| Condition of periwound skin and prevention of maceration | 56 | 44 | 0 | 0 |
| Ease of use (application and removal) | 67 | 33 | 0 | 0 |
| Conformability | 60 | 40 | 0 | 0 |
| Prevention of trauma on removal/minimisation of trauma to newly formed tissue | 58 | 40 | 0 | 2 |
| Patient comfort | 67 | 33 | 0 | 0 |
| Overall Assessment | 60 | 40 | 0 | 0 |
Discussion
The results of this post-market study are promising, with the use of ActivHeal® Aquafiber™ Extra resulting in positive healing outcomes in the majority of patients. The dressing was used to treat a variety of wounds, including leg ulcers, diabetic ulcers, pressure ulcers, post-operative surgical wounds and cavity wounds. ActivHeal® Aquafiber™ Extra maintained a moist wound environment and supported wound progression.
Wound size and depth improved over the study period. It was well tolerated by patients and clinician satisfaction with treatment was high overall. Data from the study support the use of ActivHeal® Aquafiber™ Extra dressing in a variety of wound types and clinical settings.
All the wounds treated had moderate to high levels of exudate at the start of the evaluation. Uncontrolled exudate can macerate periwound skin or even break it down (Adderley, 2008; Stephen- Haynes, 2011; Gardiner, 2012; WUWHS, 2019).
The reduction in observed levels of exudate and improvement in periwound skin during the evaluation period demonstrates that the dressing effectively absorbed exudate, which assists in preventing maceration. It has been widely reported in the literature that when the skin surrounding a wound is compromised, it can increase patient discomfort (WUWHS, 2019). The evaluation demonstrated that Aquafiber® Extra achieved high levels of comfort satisfaction.
Furthermore, the majority of dressing changes were performed to conduct routine wound assessment, rather than because of clinical need such as failure of the dressing to stay in place, leakage or strikethrough. This may have contributed to the high levels of patient satisfaction reported by clinicians. These factors added to the acceptability of the dressing for use in clinical practice and suggest this treatment has the potential to enhance patient comfort.
The results from this evaluation and in-vitro studies demonstrate the clinical effectiveness of this dressing with regards to clinical outcomes. The small number of clinicians who were dissatisfied with wound progression had possibly treated the wounds that did not progress towards healing and or the patients had other comorbidities that would affect progress during the evaluation period. The overall satisfaction levels reported by clinicians suggests that the use of ActivHeal® Aquafiber™ Extra is more than acceptable in practice.
Limitations
This evaluation included just 43 patients. Larger studies are needed to further explore whether the findings are related to the cohort or are applicable to all patients with wounds. Further studies are planned. Exudate levels are hard to assess and quantify. They can be subjective and are dependent on the judgement of the clinician assessing the wound (WUWHS, 2019).
CONCLUSION
It is important to select a product that will create an optimal wound healing environment and manage exudate, taking into consideration treatment efficacy, structural integrity and wear time. This clinical evaluation found Activheal® Aquafiber™ Extra to be an effective primary dressing for the management of both acute and chronic wounds. The results suggest that the use of Aquafiber® Extra in clinical practice is safe, effective and acceptable to practitioners and patients and provides an alternative to other gelling fibre dressings.
Declaration of Interest
This article was supported by Advanced Medical Solutions.
References
- Adderley U (2008) Wound exudate: what it is and how to manage it. Wound Essentials 3: 8–13
- Aderibigbe BA, Buyana B (2018) Alginate in wound dressings. Pharmaceutics 10(2): 42
- Chadwick P, McCardle J (2015) Exudate management using a gelling fibre dressing. Diabetic Foot Journal 18(1): 43–8
- Dowsett C (2015) Breaking the cycle of hard-to-heal wounds: balancing cost and care. Wounds International 6(2): 17–21
- Dowsett C (2012) Management of Wound Exudate. Available at: www.independentnurse.co.uk/clinical-article/management-of-woundexudate/63637/ (accessed 18.05.2020)
- Gardiner S (2012) Managing high exudate wounds, How to Guide. Wound Essentials 7(1 Suppl): 1–4
- Guest JF, Ayoub N, McIlwraith T et al (2017) Health economic burden that different wound types impose on the UK’s National Health Service. Int Wound J 14(2): 322–30
- International Best Practice Statement (2016) Optimising Patient Involvement in Wound Management. Available at: https://www.woundsinternational.com/resources/details/international-bestpractice-statement-optimising-patient-involvement-in-woundmanagement(accessed 19.05.2020)
- Jones N, Ivins N, Ebdon V et al (2017) A case series evaluating the use of a gelling fibre dressing for moderate to highly exuding wounds. Wounds UK 13(3): 72–78
- Mudge E, Orsted H (2010) Wound infection & Pain management, Made Easy. Wounds International 1(3 Suppl): S1–6
- Naik G, Harding KG (2019) Assessment of acceptability and ease of use of gelling fibre dressings in the management of heavily exuding wounds. Chronic Wound Care Management and Research 2019(6): 19–26
- NHS Business Services Authority (2018) Clinical Review: Gelling Fibre Dressings. Version 2. January 2018. Available at: https://wwwmedia.supplychain.nhs.uk/media/Clinical-Reviewfor-Gelling-Fibre-Dressings-V2-January-2018.pdf (accessed 19.05.2020)
- National Institute for Health and Care Excellence (2016) Evidence Summary. Chronic Wounds: Advanced wound dressings and antimicrobial dressings. NICE, London. Available at: https://www.nice.org.uk/advice/esmpb2/resources/chronicwounds-advanced-wound-dressings-and-antimicrobialdressingspdf-1502609570376901 (accessed 19.05.2020)
- Naude L, Ivins N (2017) Gelling fibre dressing for moderate to highly exuding wounds shows promising exudate management. Wounds International 8(2): 47–51
- Nazarko L (2018) Choosing the correct wound care dressing: an overview. Journal of Community Nursing 32(5): 42–52
- Rippon M, Waring M, Bielfeldt S (2015). An evaluation of properties related to wear time of four dressings during a fiveday period. Wounds UK 11(1): 45–54
- Rippon M, Davies P, White R (2012) Taking the trauma out of wound care: the importance of undisturbed healing. J Wound Care 21(8): 359–60, 362, 364–8
- Romanelli M, Vowden K, Weir D (2010) Exudate Management Made Easy. Wounds International 1(2 Suppl): S1–6
- Sood A, Granick MJ, Tomaselli NL (2014) Wound dressings and comparative effectiveness data. Adv Wound Care (New Rochelle) 3(8): 511–29
- Stephen-Haynes J (2011) Managing exudate and the key requirements of absorbent dressings. Br J Community Nurs 16(3 Suppl): S44–9
- Timmons J (2009) Alginates as haemostatic agents: worth revisiting? Wounds UK l 5(4): 122–5
- Walsh C, Bradford C, Krajnakova S, Knight M, Advanced Medical Solutions Ltd (2017) A Comparison of Performance Characteristics of a New High Performance Dressing Compared with a 100% CMC Fibre Based Dressing and its Reinforced Equivalent. Poster: Wounds UK, 2017. Available at: https:// epostersonline.com/wounds2017/node/604?view=true (accessed 19.05.2020)
- Waring MJ, Parsons D (2001) Physico-chemical characterisation of carboxymethylated spun cellulose fibres. Biomaterials 22(9): 903–12
- World Union of Wound Healing Societies (2019) Consensus Document. Wound exudate: Effective Assessment and Management. Available at: https://www.woundsinternational.com/resources/details/wuwhs-consensus-document-woundexudate-effective-assessment-and-management (accessed 28.05.2020)
ActivHeal® AquaFiber™ is a registers trademark of Advanced Medical Solutions Limited. All rights reserved.
CONTACT US FOR MORE INFORMATION
* Required Field
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd

