Clinical evaluation of the effect of ActivHeal Aquafiber Ag dressing
DONNA WELCH
Advanced Podiatrist, City Healthcare Partnership, Doncaster
LYNNE HEPWORTH
Lead Tissue Viability Nurse, South West Yorkshire Partnership Foundation Trust, Barnsley
SIMON BARRETT
Tissue Viability Lead, Humber NHS Foundation Trust, Hull and the East Riding of Yorkshire
JOANNE OVERFIELD
Tissue Viability Nurse, Humber NHS Foundation Trust, Hull and the East Riding of Yorkshire
REBECCA FORDER
Clinical Research Manager, Advanced Medical Solutions, Winsford
Clinical evaluation of the effect of ActivHeal Aquafiber Ag dressing
DONNA WELCH
Advanced Podiatrist, City Healthcare Partnership, Doncaster
LYNNE HEPWORTH
Lead Tissue Viability Nurse, South West Yorkshire Partnership Foundation Trust, Barnsley
SIMON BARRETT
Tissue Viability Lead, Humber NHS Foundation Trust, Hull and the East Riding of Yorkshire
JOANNE OVERFIELD
Tissue Viability Nurse, Humber NHS Foundation Trust, Hull and the East Riding of Yorkshire
REBECCA FORDER
Clinical Research Manager, Advanced Medical Solutions, Winsford

ABSTRACT
KEY WORDS
- ActivHeal Aquafiber Ag dressing
- Clinical evaluation
- Exudate management
- Moist wound environment
- High gelling fibre technology
HIGH GELLING FIBRE TECHNOLOGY
Alginates have been used within the wound care environment since the early 1940s (Clarke, 2012) and are considered to be versatile dressings, particularly for the management of wounds where there is excess exudate. Alginates like hydrofibre are applied to the wound as a fibre sheer, rope or ribbon, which provides an ideal moist environment required to support wound healing (Clarke, 2012; Hedger, 2014). Both alginates and hydrofibers are chosen for their ability to absorb exudate, creating a gel for ease of removal and ensuring it is undertaken without damaging the wound bed. They can be used on sloughy wounds to aid and facilitate autolytic debridement. They can achieve this by creating a warm, moist environment as they form a gel within the wound bed on contact with wound exudate (Hedger, 2014).
SILVER ANTIMICROBIAL DRESSINGS
Silver is a broad spectrum agent that is effective against a large number of gram positive and gram negative microorganisms, many aerobes and anaerobes and several antibiotic strains such as methicilin resistant Staphylococcus aureus (MRSA) and Vancomycin resistant Enteroccoci (VRE) (Wounds International, 2012). In addition, in vitro silver ions are thought to affect multiple sites within a negatively charged cell component, e.g. the cell wall, DNA and RNA. This disrupts the function of these cell elements and results in cell lysis and interface with electron transport, enzyme function and cell division (Wounds International, 2012). In addition, in vitro studies using experimental biofilm models suggest that silver may kill bacteria within the biofilm matrix and reduce bacterial adhesion, destabilising the matrix (Chaw et al, 2005, Percival et al, 2008). When choosing an appropriate antimicrobial dressing, the priority should be to regain control of the bacterial growth along with reducing the bacterial load. Clinicians should consider how different antimicrobial products support moist wound healing, manage exudate levels and assist in wound bed preparation (Vowden et al, 2011).
Although widespread or indiscriminate use of antimicrobial dressings prophylactically is not advocated, there is guidance to support their use in patients with a risk of infection due to comorbidities, or evidence of a history of recurrentinfection.Suchpatientsmaybeatriskof developing cellulitis, or systemic infection which has important clinical, quality of life and cost implications (Wounds UK, 2011).The International Consensus document (Wounds International, 2012) recommends that silver dressings be used initially for a two week ‘challenge period’. At the end of the two week period, the wound, the patient and the management approach should be re-evaluated. If after two weeks, the wound has:
- Improved, but there are continuing signs of infection; it may be clinically justifiable to continue to use silver dressings with regular reviews.
- Improved and there are no longer signs or symptoms of infection, the silver dressing should be discontinued.
- Not improved, the silver dressing should be discontinued and the patient reviewed and a dressing containing a different antimicrobial agent initiated, with or without systemic antibiotics (Wounds International, 2012).
AQUAFIBER AG
Figure 1. Absorbency and fluid handling capacity
Figure 2. Combined wet tensile strength
| AQUAFIBER AG | AQUACEL AG | |||||
|---|---|---|---|---|---|---|
| BACTERIA | 7 DAYS | 14 DAYS | 21 DAYS | 7 DAYS | 14 DAYS | 21 DAYS |
| MRSE | Yes | Yes | Yes | Yes | Yes | No |
| MRSA | Yes | Yes | Yes | Yes | Yes | No |
| VRE | Yes | Yes | Yes | Yes | Yes | No |
| S.pyogenes | Yes | Yes | Yes | Yes | Yes | No |
| S.epidermidis | Yes | Yes | Yes | Yes | Yes | No |
| E.Coli | Yes | Yes | Yes | Yes | Yes | Yes |
| C.Albicans | Yes | Yes | Yes | Yes | Yes | No |
| P.aeruginosa | Yes | Yes | Yes | Yes | Yes | Yes |
ActivHeal Aquafiber Ag dressings are indicated for use in the management of moderate to heavily exuding partial to full thickness wounds; post operative wounds, trauma wounds (dermal lesions, trauma injuries or incisions), leg ulcers, pressure ulcers, diabetic ulcers, graft and donor sites, cavity wounds and superficial and partial thickness burns. ActivHeal Aquafiber Ag dressing can be used under medical supervision, in the management of infected wounds or wounds in which there is an increased risk of infection.
The ActivHeal Aquafiber Ag dressing has been shown in a number of laboratory studies to have good antimicrobial activity and high absorbent capacity. It has shown to be well tolerated and is suitable for a wide range of wounds offering:
- High absorbency and total fluid handling capacity (Figure 1)
- High wet strength (Figure 2)
- Good antimicrobial activity against many common wound pathogens, including multi resistant organisms (Figure 3).
CLINICAL EVALUATION
Dressing performance was evaluated by ease of use, conformability to the wound; patient comfort during removal and wear, clinicians’ satisfaction in regards to ease of application and removal, and its ability to stay in place if the patient showered. The timescale for the evaluation was for a maximum of 10 weeks, although the dressing could be discontinued if the wound healed, was changed to implement a different treatment or if the patient requested the discontinuation. Patients over 18 years of age were included in the evaluation, if they were assessed suitable for a Silver fibre dressing; they were only excluded if they could not give informed consent or had suspected allergies to any of the dressing components. An initial assessment of each patient was undertaken and recorded, during which time the age, sex, wound type, comorbidities, past medical history of the patients were established, including wound type, duration, wound bed status, exudate level; peri wound skin condition, pain, and if the wound showed clinical signs and symptoms of infection (erythema, heat, oedema, abnormal discharge, increased level of exudate, pyrexia, friable tissue, and wound breakdown) as well as previous treatment were also recorded.
At each dressing change, the wound assessment was repeated, as well as the reason for the dressing change and performance of the dressing in terms of comfort, ease of application and removal, and additional products used. At the end of the evaluation, the clinicians were asked to rank the overall performance as either very satisfied, satisfied or not satisfied in regards to managing clinical signs and symptoms of infection, managing exudate, maintaining a moist environment, wound progression, ease of use, conformability of the dressing, patient satisfaction and overall assessment of the dressing. All data was recorded in a standardised form, and a simple analysis was planned where no statistic methods were employed.
RESULTS
Figure 4. Wound progression at the end of the clinical evaluation
At the end of the evaluation the Aquafiber Ag dressing was used on all the indicated wound types and 83% of the wounds treated improved (n=31) and showed wound improvement, progression and healing, 3% of the wounds (n=1 healed), and 8% (n=3) showed no wound progression with the wound sizes remaining the same however; the tissue types and wound aetiology showed improvement with a reduction in the percentage of necrotic and sloughy tissue in the wound bed. Of all wounds, 6% (n=2) did not have final measurements, so could not be counted. However, they had shown progression over the course of the evaluation when the measurements had been recorded (Figure 4). No wound sizes increased and there were positive changes and clear wound progression even if it was just removal of devitalised tissue. At the start of the evaluation 11% (n=4) had necrotic tissue, 5% (n=2) had sloughy tissue, 8% (n=3) had both necrotic and granulating tissue, 68% (n=25) had both sloughy and granulating tissue, and 8% (n=3) had granulating tissue. At the end of the evaluation 0% had necrotic tissue, 5% (n=2) necrotic, sloughy and granulating tissue, 0% had necrotic and granulating tissue, 0% had sloughy tissue, 54% (n=20) had sloughy and granulating tissue, 11% (n=11) had granulating tissue, 3% (n=1) had granulating and epithelising tissue and 11% (n=5) had epithelial tissue (Figure 5).
The results show that the dressing met the objective of over 70% of wounds showing improvement in wound bed condition (reduction in unhealthy tissue- slough and soft necrosis, increase in granulation and epithelial tissue) with 83% (n=31) of the wounds improving and 3% (n=1) being healed. The results show that the ActivHeal Aquafiber Ag is; enabling wound progression and providing a systematic approach to removing the barriers to healing and enhancing the effects of advanced therapies (Dowsett, 2005). Based on the data, the mean wound length and width were calculated to be 3.79 cm and 1.73 cm and the depth as 1.1cm at the start of the evaluation and 2.3 cm and 1.5 cm and the depth 0.98 cm at the end of the evaluation. Measuring a wound at the start of the treatment is seen as best practice to enable accurate assessment of the impact of a clinician’s intervention (Dowsett, 2005). Subsequent measuring can identify whether or not a wound is failing to heal or deteriorating, as the edge of the wound will not epithelise unless the wound bed is well prepared. The data demonstrates a reduction in the mean length and width of the wound at the end of the evaluation, demonstrating wound progression.
MANAGING EXUDATE
At the beginning of the evaluation, 16 of the patients on whom the ActivHeal Aquafiber Ag dressing was used as the primary dressing, were observed to have healthy periwound skin. This figure increased to 18 at the end of the study. There was also a reduction in maceration and inflamed skin and some patients were noted to have both symptoms. At the start of the evaluation, 11 patients had macerated periwound skin, reducing to 7 patients at the end of the evaluation (Figure 7). 16 patients had inflamed periwound skin at the start of the evaluation, reducing to 6 patients at the end of the evaluation. This demonstrates that the ActivHeal Aquafiber Ag dressing is effectively managing wound exudate. Those patients who still had both macerated and inflamed periwound skin were reassessed and moved to a more absorbent secondary dressing along with the ActivHeal Aquafiber Ag dressing. Although gelling fibres can absorb much of the exudate produced by heavily exuding wounds, a more absorbent secondary dressing may be required (Clark, 2012). Once the secondary dressing had been changed there was further improvement to the periwound skin.
Figure 5. Tissue types at the start and end of the clinical evaluation
Figure 6. Exudate levels at the start and end of the clinical evaluation
Figure 7. Peri wound skin levels at the start and end of the clinical evaluation
CLINICAL SIGNS OF INFECTION
Figure 8. Exudate levels at the start and end of the clinical evaluation
N.b. 33% reduction in wounds showing infection
PAIN
PERFORMANCE
- Management of exudate 100% (n=37) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Maintaining a moist wound environment 97% (n=36) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Wound progression 97% (n=36) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Reducing signs of infection 94% (n=35) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Ease of use 100% (n=37) of clinicians were ‘satisfied’ or ‘very satisfied’.
- Satisfaction on the conformability of the dressing 100% (n=37) were ‘satisfied’ or ‘very satisfied’. Patient satisfaction 95% (n=35) were ‘satisfied’ or ‘very satisfied’.
- Overall assessment of the dressing 100% (n=37) were ‘satisfied’ or ‘very satisfied’.
In respect to exudate management, maintaining a moist wound environment, reducing the signs of infection and wound progression, the above evidence substantiates the safety and performance of the dressing whilst meeting its intended performance of treating patients with infected wounds or those at high risk of infection and translates into positive outcomes for the patient and clinician. The results suggest that the ActivHeal Aquafiber Ag dressing effectively managed exudate levels, maintained a moist wound environment and reduced the clinical signs and symptoms of infection. The ActivHeal Aquafiber Ag dressing also addresses patient’s needs in terms of ease of use, conformability and patient satisfaction and comfort, which again translates into positive outcomes for the patient.
The results of the clinical follow up meets the primary and secondary objectives, providing evidence that the ActivHeal Aquafiber Ag dressing had positive healing outcomes and performance for patients with hard-to-heal wounds.
Figure 9. Percentage of clinician and patient satisfaction levels of dressing characteristics
CONCLUSION
Antimicrobial dressings are popular and used frequently in the management of infected exuding wounds, or at risk of infection. The clinical evaluation was limited in that it had uncontrolled patient numbers, however, the results of the clinical evaluation do demonstrate positive endpoints for both the primary and secondary objectives in the cohort of 37 patients. It can be concluded that the ActivHeal Aquafiber Ag dressing is safe, performs well and is efficacious in promoting healing through the management of wound exudate, maintenance of a moist wound environment as well as controlling the wound bioburden in the management of both acute and chronic wounds. The clinical evaluation provides good preliminary evidence of the clinical benefits of the ActivHeal Aquafiber Ag dressing.
CASE STUDY
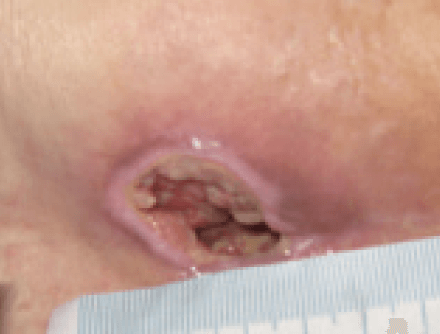
At initial assessment, the ulcer measured 3.7 cm x 3.2cm with a depth of 2cm and the wound undermined downwards towards the rectum by 3.5cm. The pressure ulcer had 50% slough and 50% granulation tissue and high exudate levels. The peri wound appeared macerated. The pressure ulcer was showing clinical signs of infection of erythema, abnormal discharge and increased levels of exudate along with a VAS score for pain at 10 (10=worst pain imaginable). The wound was showing clinical signs of infection of erythema, heat, pain, high levels of exudate and abnormal odour (Figure 10). A wound swab was taken and sent for culture and sensitivity.
The ActivHeal Aquafiber Ag dressing was selected to assist in reducing wound bioburden, absorb levels of exudate, maintain a moist wound environment, facilitate autolytic debridement and promote healing. The ActivHeal Aquafiber Ag was secured using ActivHeal Foam Contact dressing. The patient was also to be nursed on an alternating pressure relieving mattress and being repositioned every 2 hours.
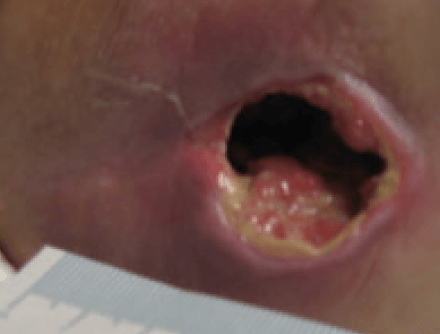
Significant progress was noted in the wound, with the wound reducing in size and showing wound progression. The wound measured 2.5 cm x 1.8 cm with a depth of 2 cm. The undermined areas had reduced to 2.5cm. There was new granulation tissue visible and epithelialisation was evident at the wound margin and a reduction in the amount of slough. Exudate levels remained high however the peri wound was healthy and no longer macerated (Figure 11). The patient was expressing high levels of pain, a VAS score of 10. The wound swab results showed heavy growth of mixed anaerobes and proteus species sensitive to Metronidazole, therefore the patient was commenced on a 7 day course of antibiotics.
The same dressing regime was continued as the wound was progressing and reducing in size along with the facilitation of debridement of devitalised tissue despite the high bacterial burden within the wound.
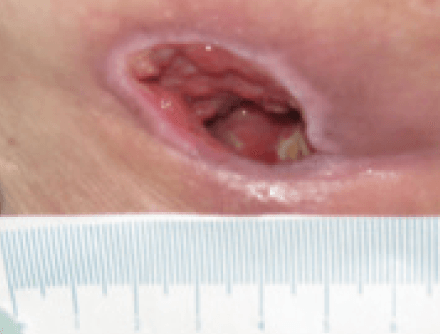
Further progress was noted in the wound with a continued reduction in slough, promotion of granulating tissue and epithelial tissue apparent at the wound edges. The wound had 5% sloughy tissue, 90% granulation tissue and 5% epithelial tissue. The wound continued to have high levels of exudate however, the peri wound skin was normal and showed no signs of maceration. The wounds measured 2.3 cm x 2 cm with a depth of 2cm (Figure 12). The clinical signs of infection were reducing, with only abnormal odour apparent, and the patients pain level had reduced to 6 using a visual analogue scale. The same dressing regime continued.
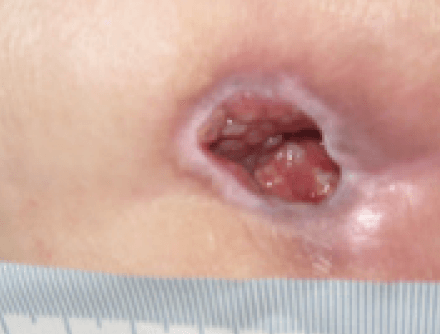
At week 3 the wound was reviewed and had continued to improve. The wound measured 2cm x 2cm with a depth of 2cm. The wound no longer contained sloughy tissue but had 95% granulation tissue and 5% epithelial tissue. Exudate levels remained high but all the clinical signs of infection were no longer apparent. The patient’s pain level had also reduced to 0 using a visual analogue scale. Following a full reassessment by the tissue viability nurse, the ActivHeal Aquafiber Ag was discontinued and moved to the use of Negative Pressure Wound Therapy (Figure 13).
CONCLUSION
The ActivHeal Aquafiber Ag dressing was found to be an appropriate dressing in the management of the infected category 4 pressure ulcer with high exudate levels and bacterial bioburden within the wound. Despite this, the wound improved over the 3 weeks of treatment. Granulation tissue was evident in just a few days. Sloughy tissue was also debrided very quickly. The dressing produced very positive patient outcomes. The correct dressing choice in this case enabled the patient to be managed quickly and effectively without an overly long treatment time and assisted in the management of clinical indications of exudate management, reducing wound bioburden along with being safe and acceptable to the patient. The patients pain score reduced throughout the use of the dressing. The case study illustrates the importance of a holistic approach when caring for a patient with a challenging wound and ensuring that the correct diagnosis is made based upon a thorough assessment ensuring good clinical outcomes for the patient.
References
- Advanced Medical Solutions (2015) Data on File. LD89-17
- Clarke M (2012) Rediscovering alginate dressings. Wounds International 3(2): 24–8
- Chaw K, Manimaran M, Tay F (2005) Role of silver ions in destabilisation of
intermolecular adhesion forces measured by atomic force microscopy in Staphylococcus epidermis biofilms. Antimicrob Agents Chemother 49(12): 48–59 - Dowsett C, Newton H (2005) Wound Bed Preparation: TIME in Practice. Available at: www.wounds-uk.com/pdf/content_9029.pdf (accessed on 18.10.2017)
- European Pressure Ulcer Advisory Panel (2014) Prevention and Treatment ofPressureUlcers:ClinicalPracticeGuideline.HaeslerE(ed)Cambridge Media, Perth, Australia
- European Wound Management Association (2006) Association Position Document: Identifying Criteria for Wound Infection. Available at: http://ewma.org/ leadmin/user_upload/EWMA.org/Position_ documents_2002-2008/English_pos_doc_ nal.pdf (accessed on 18.10.2017)
- Gri n J (2014) E ective exudate management improves patient well being. Journal of Community Nursing 28 (5 Suppl): 16–6
- Hedger C (2014) Choosing the appropriate dressing: Alginate and Hydro ber. Wounds Essentials 9( 1): 29–33
- PercivalS,BowlerP,WoodsE(2008)Assessingthee ectofanantimicrobial wound dressings on biofilms. Wound Repair Regen. 16 (1): 52–7
- Rivas C, Rivas Y, Valero K (2014) E ectiveness of the Dressing with Silver Hydro ber vs Calci Alginate in Ulcer in Healing in Diabetic Foot. Published esis
- Timmons J, Cooper P (2008) How to systematically assess a patient with a cavity wound. Wounds UK Vol 4(2): 4–10
- Vowden P, Vowden K, Carville K (2011) Antimicrobial Dressings Made Easy. Available at: www.woundsinternational.com/made-easys/view/ antimicrobial-dressings-made-easy-1 (accessed 18.10.2017)
- Wounds International (2012) International Consensus Appropriate Use of Silver Dressings in Wounds. An Expert Working Group Review. Available at: www.woundsinternational.com/media/issues/567/ les/ content_10381.pdf (accessed on 18.10.2017)
- World Union of Wound Healing Societies (2007) Principles of Best Practice: Wound Exudate and the Role of Wound Dressings. A Consensus Document. Available at: www.woundsinternational.com/media/ issues/82/ les/content_42.pdf (accessed on 18.10.2017)
- Wounds UK (2011) Best Practice Statement: e Use of Topical Antiseptics/ Antimicrobials in Wound Management. Available at: http://bit. ly/2zceoi1 (accessed 18.10.2017)
CONTACT US FOR MORE INFORMATION
* Required Field
Discover ActivHeal®
Social Media
Our Product Range
AMS Group
ActivHeal®, its logo and the Advanced Medical Solutions logos are registered trademarks of Advanced Medical Solutions Ltd.
Copyright © Advanced Medical Solutions Limited | Design by Lumisi Ltd