STRONG WHEN YOU NEED IT
GENTLE WHEN YOU NEED IT MORE.
ACTIVHEAL® SILICONE FOAM
FREE TWO WEEK EVALUATION
STRONG WHEN YOU NEED IT
GENTLE WHEN YOU NEED IT MORE.
ACTIVHEAL® SILICONE FOAM
FREE TWO WEEK EVALUATION
Post-market clinical evaluation of the safety and performance of ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite dressings
This post-market evaluation assessed the performance of the ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite dressings and gathered feedback from clinicians using both dressings to treat a variety of wounds. The primary outcomes were progression to healing through management of exudate and maintenance of a moist wound healing environment. The evaluation took place at 1 site within Poland, where 53 patients were treated according to ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite dressings’ instructions for use, and standard local practice. Data was collected at every dressing change. The results showed that both ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite Dressings had positive effects on exudate levels, periwound condition and tissue type(s) within the wound bed. The majority of wounds reduced in size during the evaluation period and twelve completely healed. Clinical and patient satisfaction was high. No adverse events were reported. The evaluation shows ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite Dressings used in clinical practice are safe, effective and acceptable for use to both practitioners and patients.
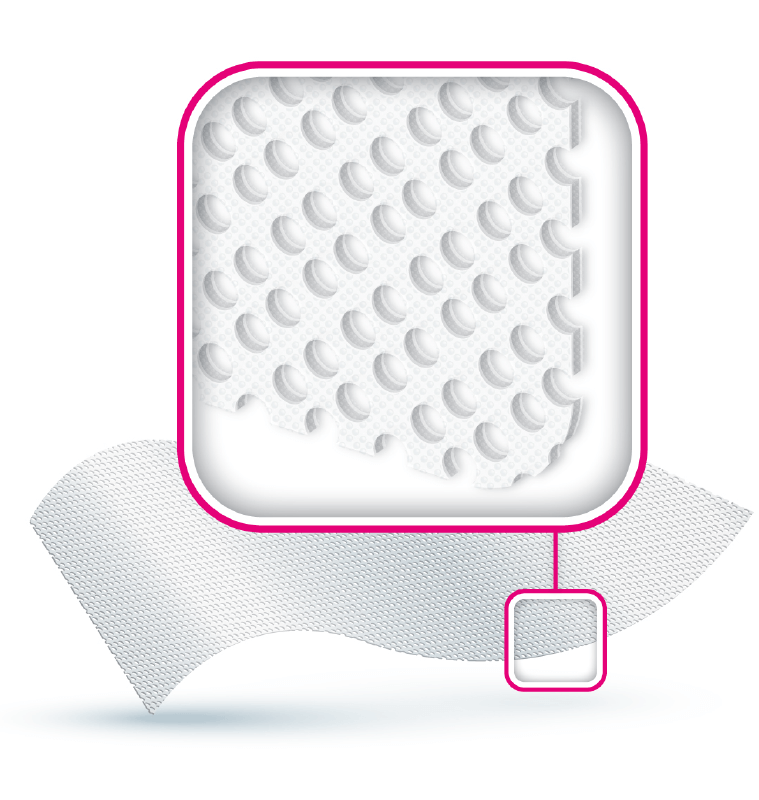
ACTIVHEAL® SILICONE FOAM DRESSINGS HAVE A GENTLE BUT SECURE ADHESION WITH MINIMAL EPIDERMAL STRIPPING OR PAIN ON REMOVAL.
FEATURES // Highly conformable // Excellent Absorption of exudate // Can remain in situ for up to 7 days // Minimises epidermal stripping // Promotes healing through a moist wound environment // Reduces pain at dressing changes // Protects the periwound // For moderate to heavily exuding wounds //
PERFORMANCE
Total Fluid Handling Performance1
Be confident this foam can handle patient exudate.

Peel adhesion over 7 days2
Secure but pain free removal.

PRODUCT RANGE
- EVERYDAY: Silicone Foam Border & Silicone Foam Non-Border
- SPECIALIST: Silicone Sacral
INDICATIONS
- Pressure ulcers
- Diabetic ulcers
- Venous and arterial leg ulcers
- Surgical wounds
- Trauma wounds
- Superficial and partial thickness burns
- Skin Abrasions
- Use as a secondary dressing on cavity wounds
ActivHeal® Silicone Adhesive Border Foam Dressing may be used as part of a prophylactic therapy for skin pressure ulceration/ skin damage*.
WOUND TYPES
Moderate > Heavy Exudate
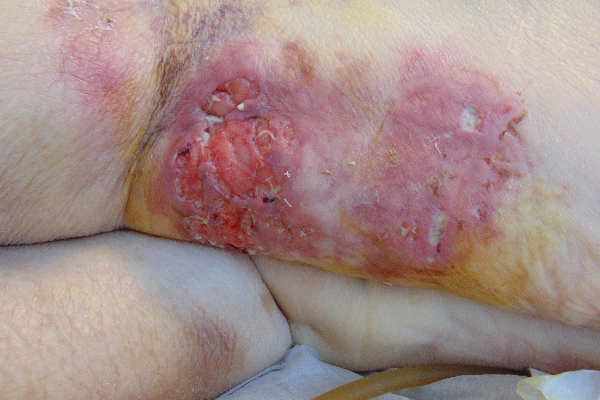
CASE STUDY

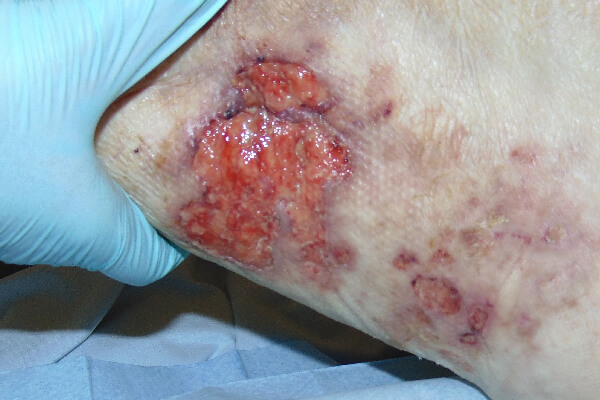
CASE STUDY
SIZES & CODES
| ACTIVHEAL® | SILICONE FOAM BORDER | SILICONE FOAM NON-BORDER | |||||
|---|---|---|---|---|---|---|---|
| SIZE (CM) | QTY | PRODUCT CODE | DT PIP CODE | NHS SUPPLY CHAIN | PRODUCT CODE | DT PIP CODE | NHS SUPPLY CHAIN |
| 5x5 | 10 | 9001548 | 402-5383 | ELA847 | |||
| 7.5x7.5 | 10 | 9001470 | 402-5318 | ELA843 | 9001555 | 402-5391 | ELA834 |
| 10x10 | 10 | 9001487 | 402-5326 | ELA838 | 9001562 | 402-5417 | ELA844 |
| 12.5x12.5 | 10 | 9001494 | 402-5334 | ELA840 | |||
| 10x20 | 10 | 9001500 | 402-5342 | ELA839 | 9001579 | 402-5441 | ELA833 |
| 15x15 | 10 | 9001517 | 402-5359 | ELA841 | 9001449 | 402-5425 | ELA845 |
| 20x20 | 10 | 9001524 | 402-5367 | ELA842 | 9001586 | 402-5433 | ELA846 |
| Sacral | 10 | 9001531 | 402-5375 | ELA848 | |||
*ActivHeal® Silicone Adhesive Border Foam UK/EU/ under MDR, and USA only
References
1. LD065-14
2. P3139R
Allevyn Gentle is a registered trademark of Smith & Nephew.
STRONG WHEN YOU NEED IT
GENTLE WHEN YOU NEED IT MORE.
ACTIVHEAL® SILICONE WOUND CONTACT LAYER
FREE TWO WEEK EVALUATION
STRONG WHEN YOU NEED IT
GENTLE WHEN YOU NEED IT MORE.
ACTIVHEAL® SILICONE WOUND CONTACT LAYER
FREE TWO WEEK EVALUATION
ACTIVHEAL® SILICONE WOUND CONTACT LAYER REDUCES THE DRESSING FROM STICKING TO THE WOUND AND MINIMISES PAIN DURING DRESSING CHANGES.
FEATURES // HIGHLY CONFORMABLE // ALLOWS PASSAGE OF EXUDATE // CAN REMAIN IN SITU FOR UP 14 DAYS // PROMOTES HEALING THROUGH A MOIST WOUND ENVIRONMENT // PROTECTIVE LAYER ON NON EXUDING WOUNDS SUCH AS BLISTERS AND FRAGILE SKIN TEARS // MINIMISES EPIDERMAL STRIPPING // REDUCES PAIN AT DRESSING CHANGES // PROTECTS THE PERIWOUND //
PERFORMANCE
Conformability (kgfcm-1)2 1
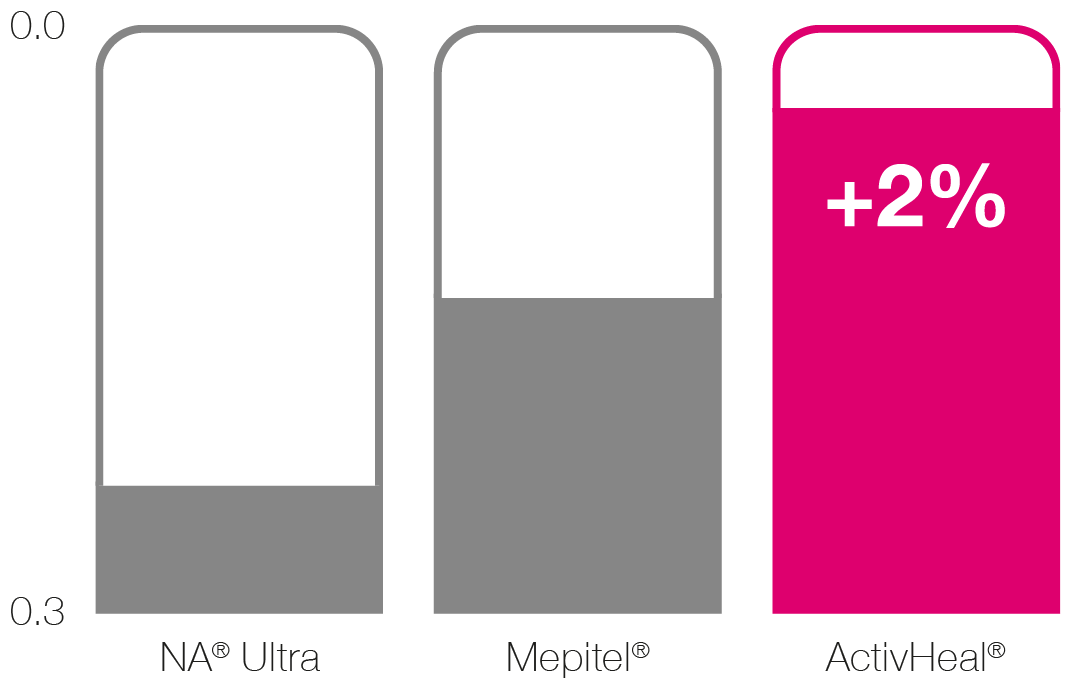
█ NA® Ultra
█ Mepitel®
█ ActivHeal® Silicone
Wound Contact Layer
PRODUCT RANGE
- EVERYDAY: Silicone Wound Contact Layer Sheet
INDICATIONS
- Skin tears
- Partial thickness skin grafts
- Surgical incisions
- Partial thickness burns
- Venous ulcers
- Pressure ulcers
- Arterial ulcers
WOUND TYPES
Nil > Moderate > Heavy Exudate
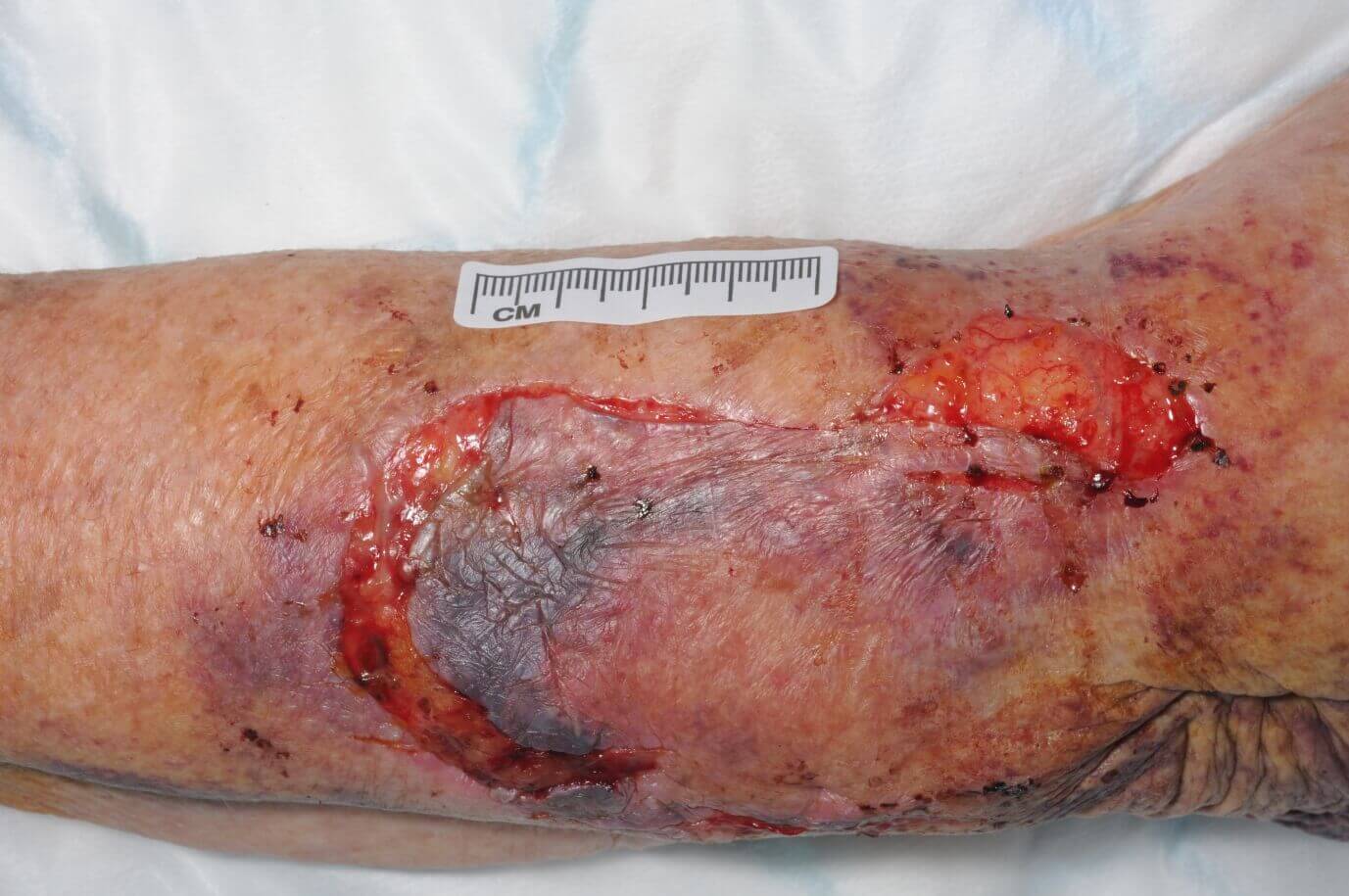
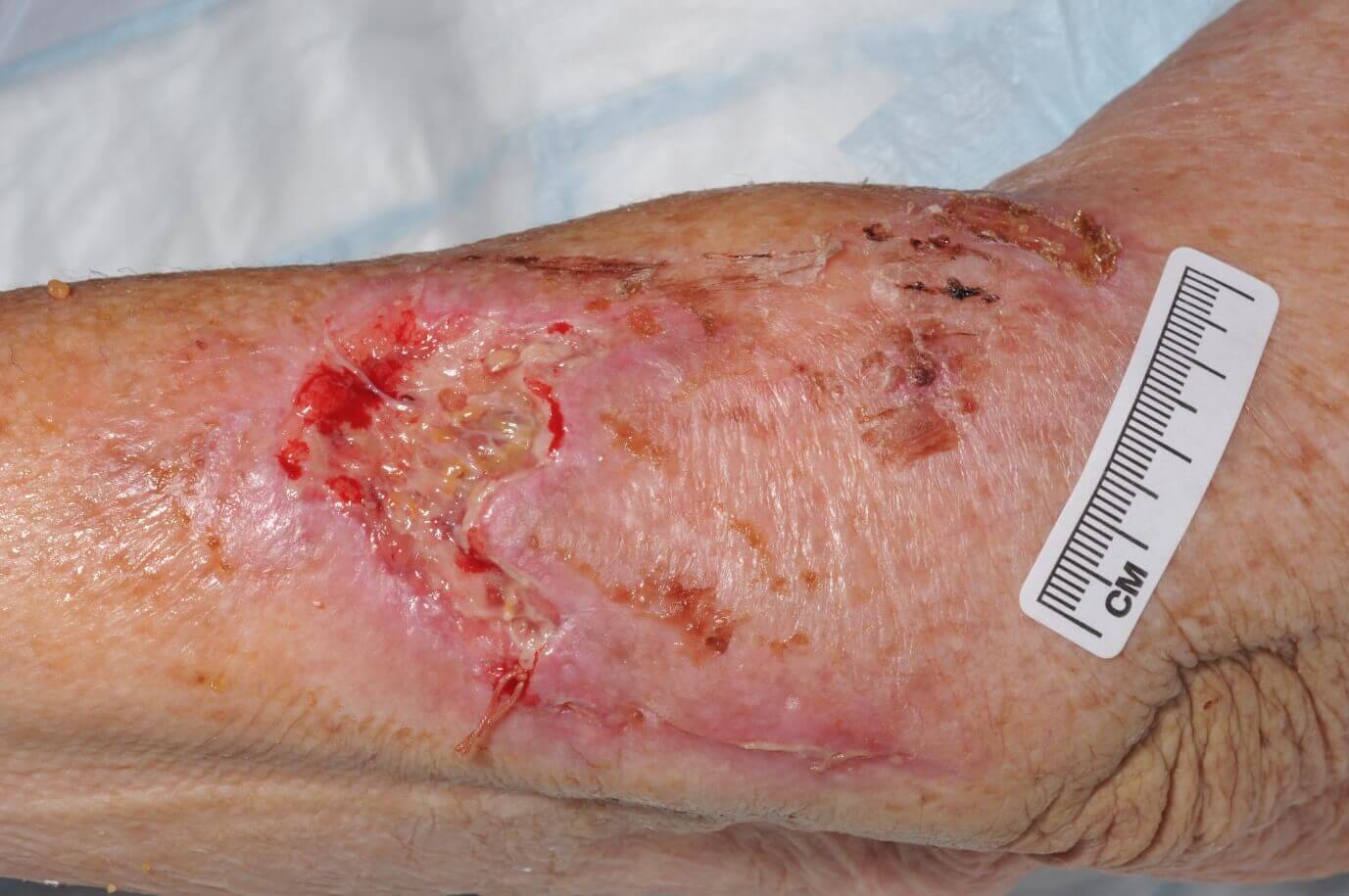
CASE STUDY

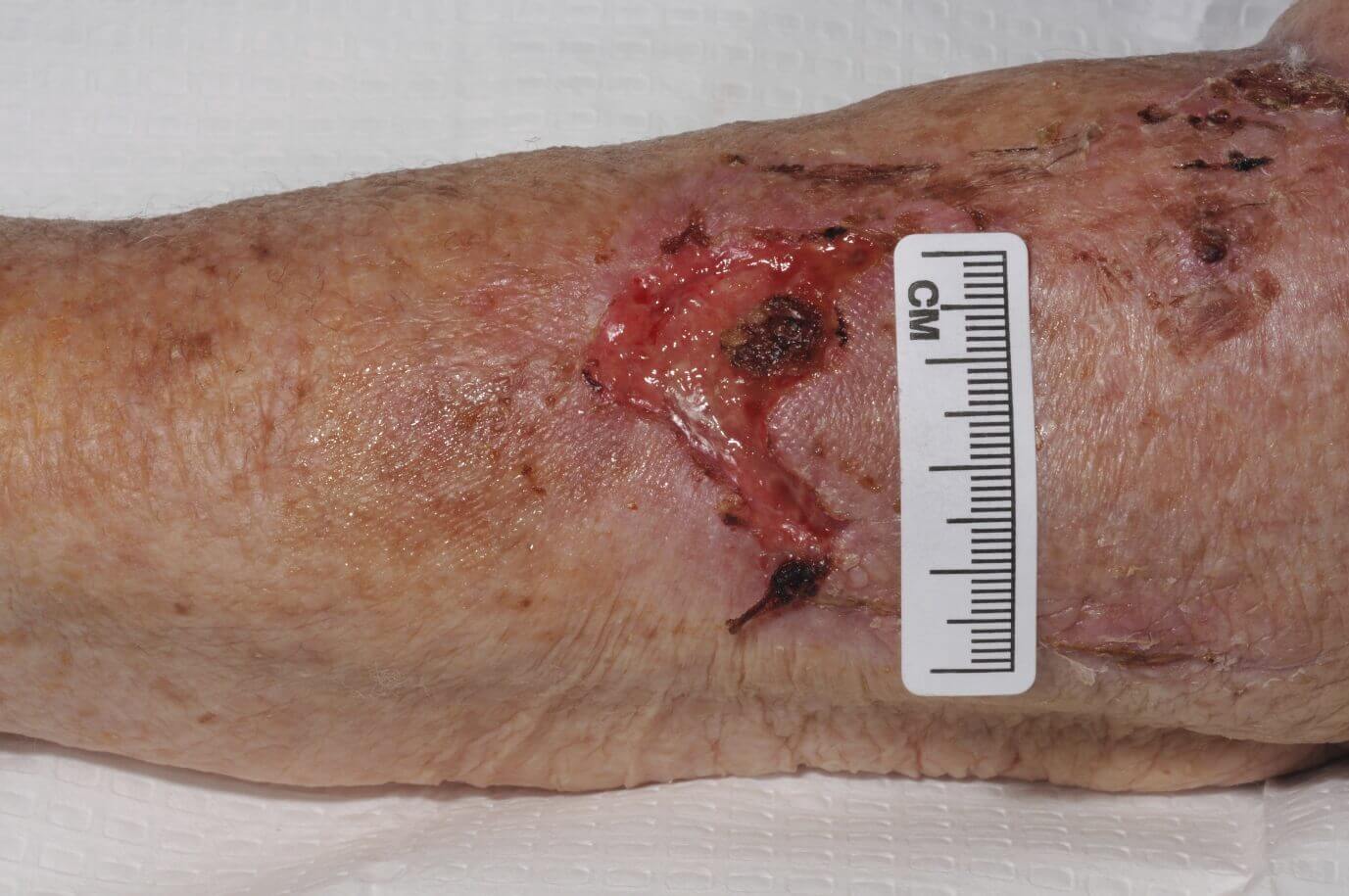
FREE TWO WEEK EVALUATION
ACTIVELY TRY SILICONE FOAM OR SILICONE WOUND CONTACT LAYER.
*Health care professionals only
SIZES & CODES
| ACTIVHEAL® SILICONE WOUND CONTACT LAYER | ||||
|---|---|---|---|---|
| SIZE (CM) | QTY | PRODUCT CODE | NHS SUPPLY CHAIN | DT PIP CODE |
| 5x7.5 | 10 | 9001241 | ELA849 | 399-7459 |
| 10x10 | 10 | 9001258 | ELA835 | 399-7442 |
| 10x20 | 10 | 9001265 | ELA836 | 399-7467 |
| 15x15 | 10 | 9001272 | ELA837 | 399-7475 |
References
1. Barrett,S.(2012) BJN 21 (21):1271-7.
2. Chart reference AMS data on file P2772
3. Hampton, S. (2010) BJN 19 (6) S30-3.
4. Carville,K. Lewin,G. Newall, N. Haslehurst, P. Michael, R. Santmaria, N. Roberts,P. (2007) STAR: A Consensus for Skin Classification. Primary Intention. 15(1) 18-28.
Mepitel® is a trademark of Molnlycke, NA Ultra is a trademark of Acelity®.
DOWNLOADS
ActivHeal® Silicone Foam
Factsheet (PDF – 890kb)
Post-market clinical evaluation of the safety and performance of ActivHeal® Silicone Foam and ActivHeal® Silicone Foam Lite dressings
Article (PDF – 222kb)
CONTACT US FOR MORE INFORMATION
Discover ActivHeal®
Social Media
Our Product Range
AMS Group